AIMP1-Derived Peptide Secreted from Hair Follicle Stem Cells Promotes Hair Growth by Activating Dermal Papilla Cells
- PMID: 39494335
- PMCID: PMC11528461
- DOI: 10.7150/ijbs.101127
AIMP1-Derived Peptide Secreted from Hair Follicle Stem Cells Promotes Hair Growth by Activating Dermal Papilla Cells
Abstract
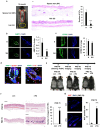
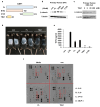
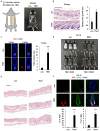
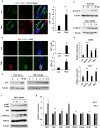
Hair follicle stem cells (HFSCs) and dermal papilla cells (DPCs) are crucial in the biogenesis and maintenance of hair follicles (HFs). This study demonstrated that a fragment derived from aminoacyl-tRNA synthetase-interacting multifunctional protein1 (AIMP1) secreted from HFSCs activated DPCs and maintained HF homeostasis. A histological analysis revealed that AIMP1 levels in HF decreased with hair loss. Hair regrowth in AIMP1-induced mice was faster than in non-induced mice. Deletion mapping revealed 41 amino acids (TN41, aa 6-46) as the active region of AIMP1. The N-terminal peptide fragment of AIMP1 generated by MMP1 was secreted from Wnt-treated HFSCs to activate DPCs. TN41 activated Akt and ERK, increased β-catenin, and enhanced DPC activation. TN41 promoted hair shaft elongation in cultured human HFs and improved the hair-inducing activity of cultured DPC spheroids. Our findings suggest that the AIMP1 fragment secreted from HFSCs stimulates active hair regrowth through activating DPCs.
Keywords: AIMP1; dermal papilla cell; hair follicle stem cell; hair growth.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Ji J, Ho BS, Qian G, Xie XM, Bigliardi PL, Bigliardi-Qi M. Aging in hair follicle stem cells and niche microenvironment. J Dermatol. 2017;44:1097–104. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

