Altered Spike Immunoglobulin G Fc N-Linked Glycans Are Associated With Hyperinflammatory State in Adult Coronavirus Disease 2019 and Multisystem Inflammatory Syndrome in Children
- PMID: 39494457
- PMCID: PMC11528514
- DOI: 10.1093/ofid/ofae626
Altered Spike Immunoglobulin G Fc N-Linked Glycans Are Associated With Hyperinflammatory State in Adult Coronavirus Disease 2019 and Multisystem Inflammatory Syndrome in Children
Abstract
Background: Severe coronavirus disease 2019 (COVID-19) and multisystem inflammatory syndrome (MIS-C) are characterized by excessive inflammatory cytokines/chemokines. In adults, disease severity is associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific immunoglobulin G (IgG) Fc afucosylation, which induces proinflammatory cytokine secretion from innate immune cells. This study aimed to define spike IgG Fc glycosylation following SARS-CoV-2 infection in adults and children and following SARS-CoV-2 vaccination in adults and the relationships between glycan modifications and cytokines/chemokines.
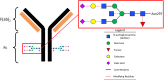
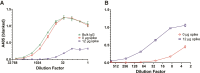
Methods: We analyzed longitudinal (n = 146) and cross-sectional (n = 49) serum/plasma samples from adult and pediatric COVID-19 patients, MIS-C patients, adult vaccinees, and adult and pediatric controls. We developed methods for characterizing bulk and spike IgG Fc glycosylation by capillary electrophoresis and measured levels of 10 inflammatory cytokines/chemokines by multiplexed enzyme-linked immunosorbent assay.
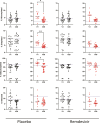
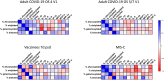
Results: Spike IgG was more afucosylated than bulk IgG during acute adult COVID-19 and MIS-C. We observed an opposite trend following vaccination, but it was not significant. Spike IgG was more galactosylated and sialylated and less bisected than bulk IgG during adult COVID-19, with similar trends observed during pediatric COVID-19/MIS-C and following SARS-CoV-2 vaccination. Spike IgG glycosylation changed with time following adult COVID-19 or vaccination. Afucosylated spike IgG exhibited inverse and positive correlations with inflammatory markers in MIS-C and following vaccination, respectively; galactosylated and sialylated spike IgG inversely correlated with proinflammatory cytokines in adult COVID-19 and MIS-C; and bisected spike IgG positively correlated with inflammatory cytokines/chemokines in multiple groups.
Conclusions: We identified previously undescribed relationships between spike IgG glycan modifications and inflammatory cytokines/chemokines that expand our understanding of IgG glycosylation changes that may impact COVID-19 and MIS-C immunopathology.
Keywords: COVID-19; Fc; MIS-C; antibody glycosylation; inflammation.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. J. A. is currently employed by Moderna and owns stock or stock options in Moderna. C. A. R. has received grants or contracts to Emory from BioFire Inc, GSK, MedImmune, Micron, Merck, Novavax, PaxVax, Regeneron, Pfizer, Sanofi-Pasteur, Janssen, Moderna, NIH, and CDC; is a co-inventor of patented respiratory syncytial virus (RSV) vaccine technology that has been licensed to Meissa Vaccines Inc and has received royalties from Meissa Vaccines Inc; and has patents pending or issued: “Chimeric RSV, Immunogenic Compositions, and Methods of Use,” International PCT Application No. PCT/US2016/058976, filed 28 December 2016 by Emory University and “RSV Live-Attenuated Vaccine Candidates with Deleted G-Protein Mucin Domains,” US Patent Application No. 63/411,251, filed 29 September 2022 by Emory University. N. R. has received grants or contracts to Emory from NIH, Merck, Sanofi, Pfizer, Vaccine Company, Immorna, Quidel, and Lilly; has received consulting fees from Krog; has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Virology Education; has received support for attending meetings and/or travel from Sanofi and Moderna; has participated in data safety monitoring boards for Emmes, ICON, Biomedical Advanced Research and Development Authority, CyanVac, and Micron and advisory boards for Moderna, Sanofi, Seqirus, and Pfizer; has held advisory roles for the Antibacterial Resistance Leadership Group, TMRC, and CDC-Pertussis challenge; is Associate Editor of Clinical Infectious Diseases; and has received equipment, materials, drugs, medical writing, gifts, or other services to Emory from Georgia Research Alliance. E. M. S. has received grants from NIH, JC Kennedy Foundation, and Georgia Clinical & Translational Science Alliance; has received materials from Merck; has received honoraria for lectures/presentations from Merck; and has patents pending or issued: “Anti-ALPP/ALPP2 Antibodies,” US Patent Provisional Application Nos. 63/162,635, 63/301,574, and 18/282,232 filed 18 March 2021, 21 January 2022, and 15 September 2023 by Seagen, “Anti-ALPP/ALPP2 Antibodies,” International PCT Application No. PCT/US2022/020697 filed 17 March 2022 by Seagen, “Antibodies that Bind CD228,” US Patent Provisional Application No. 63/408,605, filed 21 September 2022 by Seagen, “Novel Fusion Protein Specific for CD137 and CD228,” US Patent Provisional Application Nos. 63/408,634, 63/413,174, and 63/496,463 filed 21 September 2022, 4 October 2022, and 17 April 2023 by Seagen. All other authors report no potential conflicts.
Figures


Update of
-
Altered spike IgG Fc N-linked glycans are associated with hyperinflammatory state in adult COVID and Multisystem Inflammatory Syndrome in Children.medRxiv [Preprint]. 2024 Jul 14:2024.07.14.24310381. doi: 10.1101/2024.07.14.24310381. medRxiv. 2024. Update in: Open Forum Infect Dis. 2024 Oct 16;11(11):ofae626. doi: 10.1093/ofid/ofae626. PMID: 39040211 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

