Intravenous immunoglobulin‑based adjuvant therapy for severe fever with thrombocytopenia syndrome: A single‑center retrospective cohort study
- PMID: 39494463
- PMCID: PMC11600480
- DOI: 10.1002/jmv.70017
Intravenous immunoglobulin‑based adjuvant therapy for severe fever with thrombocytopenia syndrome: A single‑center retrospective cohort study
Abstract
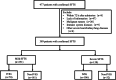
Intravenous immunoglobulin (IVIG) is frequently administered to patients with severe fever with thrombocytopenia syndrome (SFTS), particularly those with severe manifestations, although its efficacy remains controversial. The study retrospectively analyzed the effects of IVIG administration on SFTS patients in both mild and severe groups. The primary outcome measure was 28-day mortality. Inverse probability of treatment weighting (IPTW) with propensity score was used to account for baseline confounders. A total of SFTS patients with complete data enrolled from January 1, 2015, to August 1, 2023. Death at 28 days occurred for 68 (17.5%) patients. By unadjusted analysis, no difference was observed for 28-day mortality between the IVIG and non-IVIG groups in both the mild and severe groups. Similar results were found by propensity score matching and by IPTW analysis. Although IVIG is frequently used as adjuvant therapy for severe SFTS patients, no significant association was observed between IVIG treatment and reduced mortality in this patient population.
Keywords: intravenous immunoglobulin; mild and severe group; mortality; severe fever with thrombocytopenia syndrome.
© 2024 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Saijo M. Pathophysiology of severe fever with thrombocytopenia syndrome and development of specific antiviral therapy. J Infect Chemother. 2018;24(10):773‐781. - PubMed
-
- Guo CT, Lu QB, Ding SJ, et al. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome (SFTS) in China: an integrated data analysis. Epidemiol Infect. 2016;144(6):1345‐1354. - PubMed
-
- Li H, Lu QB, Xing B, et al. Epidemiological and clinical features of laboratory‐diagnosed severe fever with thrombocytopenia syndrome in China, 2011‐17: a prospective observational study. Lancet Infect Dis. 2018;18(10):1127‐1137. - PubMed
-
- Cui N, Liu R, Lu QB, et al. Severe fever with thrombocytopenia syndrome bunyavirus‐related human encephalitis. J Infect. 2015;70(1):52‐59. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources