Very Low-Intensity Ultrasound Facilitates Glymphatic Influx and Clearance via Modulation of the TRPV4-AQP4 Pathway
- PMID: 39494466
- PMCID: PMC11653672
- DOI: 10.1002/advs.202401039
Very Low-Intensity Ultrasound Facilitates Glymphatic Influx and Clearance via Modulation of the TRPV4-AQP4 Pathway
Abstract
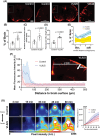
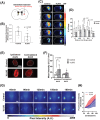
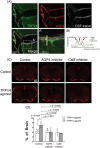
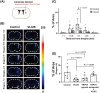
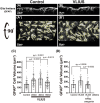
Recently, the glymphatic system has been proposed as a mechanism for waste clearance from the brain parenchyma. Glymphatic dysfunction has previously been shown to be associated with several neurological diseases, including Alzheimer's disease, traumatic brain injury, and stroke. As such, it may serve as an important target for therapeutic interventions. In the present study, very low-intensity ultrasound (VLIUS) (center frequency, 1 MHz; pulse repetition frequency, 1 kHz; duty factor, 1%; spatial peak temporal average intensity [Ispta] = 3.68 mW cm2; and duration, 5 min) is found to significantly enhance the influx of cerebrospinal fluid tracers into the paravascular spaces of the brain, and further facilitate interstitial substance clearance from the brain parenchyma, including exogenous β-amyloid. Notably, no evidence of brain damage is observed following VLIUS stimulation. VLIUS may enhance glymphatic influx via the transient receptor potential vanilloid-4-aquaporin-4 pathway in astrocytes. This mechanism may provide insights into VLIUS-regulated glymphatic function that modifies the natural course of central nervous system disorders related to waste clearance dysfunction.
Keywords: aquaporin‐4; glymphatic; transient receptor potential vanilloid‐4; ultrasound; β‐amyloid.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., Benveniste H., Vates G. E., Deane R., Goldman S. A., Nagelhus E. A., Nedergaard M., Sci. Transl. Med. 2012, 4, 147ra111; - PMC - PubMed
- b) Iliff J. J., Lee H., Yu M., Feng T., Logan J., Nedergaard M., Benveniste H., J. Clin. Invest. 2013, 123, 1299; - PMC - PubMed
- c) Nedergaard M., Science. 2013, 340, 1529; - PMC - PubMed
- d) Xie L., Kang H., Xu Q., Chen M. J., Liao Y., Thiyagarajan M., O'Donnell J., Christensen D. J., Nicholson C., Iliff J. J., Takano T., Deane R., Nedergaard M., Science. 2013, 342, 373; - PMC - PubMed
- e) Plog B. A., Dashnaw M. L., Hitomi E., Peng W., Liao Y., Lou N., Deane R., Nedergaard M., J. Neurosci. 2015, 35, 518. - PMC - PubMed
-
- a) Peng W., Achariyar T. M., Li B., Liao Y., Mestre H., Hitomi E., Regan S., Kasper T., Peng S., Ding F., Benveniste H., Nedergaard M., Deane R., Neurobiol. Dis. 2016, 93, 215; - PMC - PubMed
- b) Reeves B. C., Karimy J. K., Kundishora A. J., Mestre H., Cerci H. M., Matouk C., Alper S. L., Lundgaard I., Nedergaard M., Kahle K. T., Trends Mol. Med. 2020, 26, 285. - PMC - PubMed
-
- a) Mestre H., Du T., Sweeney A. M., Liu G., Samson A. J., Peng W., Mortensen K. N., Staeger F. F., Bork P. A. R., Bashford L., Toro E. R., Tithof J., Kelley D. H., Thomas J. H., Hjorth P. G., Martens E. A., Mehta R. I., Solis O., Blinder P., Kleinfeld D., Hirase H., Mori Y., Nedergaard M., Science. 2020, 367, aax7171; - PMC - PubMed
- b) Goulay R., Flament J., Gauberti M., Naveau M., Pasquet N., Gakuba C., Emery E., Hantraye P., Vivien D., Aron‐Badin R., Gaberel T., Stroke. 2017, 48, 2301. - PubMed
MeSH terms
Substances
Grants and funding
- 110-BIH009/National Taiwan University Hospital Hsin-Chu Branch
- 111-HCH003/National Taiwan University Hospital Hsin-Chu Branch
- NHRI-111-B01/National Taiwan University Hospital Hsin-Chu Branch
- 112-S0155/National Taiwan University Hospital
- BN-111-PP-03/National Health Research Institutes
- BN-112-PP-03/National Health Research Institutes
- 108-2321-B-002-061-MY2/National Science and Technology Council
- 109-2314-B-002-109-MY3/National Science and Technology Council
- 110-2314-B-002-070/National Science and Technology Council
- 111-2314-B-002-164-MY2/National Science and Technology Council
- 112-2314-B-002-142-MY3/National Science and Technology Council
- 112-2321-B-002-024/National Science and Technology Council
- 113-2314-B-002-214/National Science and Technology Council
- 113-2321-B-002-033/National Science and Technology Council
LinkOut - more resources
Full Text Sources
Research Materials
