Patient-Initiated Nationwide Survey on Testing for Actionable Oncogenic Drivers in Non-Small Cell Lung Cancer in Japan
- PMID: 39494523
- PMCID: PMC11532810
- DOI: 10.1002/cam4.70375
Patient-Initiated Nationwide Survey on Testing for Actionable Oncogenic Drivers in Non-Small Cell Lung Cancer in Japan
Abstract
Background: Previous reports indicated still low implementation rates of multigene testing for advanced non-small cell lung cancer (NSCLC) in Japan.
Methods: This is a retrospective study launched at the initiative of lung cancer patients. Patients with stage IV NSCLC from January 2019 to December 2022 were investigated for testing of 8 actionable oncogenic drivers with targeted therapies available as of 2022.
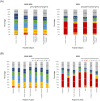
Results: A total of 15,719 patients were included. Between 2019 and 2022, the percentage of patients who were not tested for any actionable oncogenic drivers remained the same, ranging from 21.5% to 33.1%. However, since late 2021, the percentage of patients tested for five or more actionable oncogenic drivers has increased. Across hospital categories and regions, the number of actionable oncogenic drivers tested was similar.
Conclusions: This patient-initiated national survey in Japan reveals the recent nationwide increase in testing rates for actionable oncogenic drivers in Advanced NSCLC.
Keywords: actionable oncogenic drivers; diagnosis procedure combination; multigene testing; non‐small cell lung cancer; targeted therapy.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Satoshi Ikeda received honoraria from AstraZeneca, Bristol Myers Squibb, Ono, Taiho, Chugai, Boehringer Ingelheim, Eli Lilly, Takeda, Pfizer, MSD, Amgen, and Novartis; research funding from AstraZeneca and Chugai; and took on a consulting or advisory roles for AstraZeneca, Chugai and Daiichi Sankyo. Kazuo Hasegawa, Kenta Kachi, Akihiro Yanagisawa, Sachiko Kawakami, Shinsuke Hamasaki, Sachiko Watanabe, and Aki Yoshikawa have no conflicts of interest to declare. Takayuki Takahama received honoraria from AstraZeneca, Chugai, Roche Diagnostics, and MSD; research funding from Takeda and Pfizer. Kazuhiko Nakagawa received honoraria from AstraZeneca, Ono, Chugai, Boehringer Ingelheim, Taiho, Takeda, MSD, Merck, Bayer, Nippon Kayaku, Amgen. Japan Clinical Research Operations, Taiyo Pharma, Eli Lilly, Pfizer, Novartis, Daiichi Sankyo, Bristol Myers Squibb, Janssen, Otsuka, Hisamitsu, CMIC ShiftZero, CMIC Co. Ltd., Incyte biosciences, M3, Inc., Global Health Consulting Japan, YODOSHA, Medical Mobile Communications, Life Technologies and Neo Communication; research funding from AstraZeneca, Chugai, Ono, Daiichi Sankyo, Boehringer Ingelheim, Taiho, IQVIA, EPS Corporation, Bayer, MSD, Otsuka, EPS International, PRA Health Science, Labcorp Development Japan, GlaxoSmithKline, Mochida Pharmaceutical, Japan Clinical Research Operations, Sanofi, Medical Research Support, SYNEOS HEALTH, Nippon Kayaku, Mebix, Janssen, Eli Lilly, Amgen, Novartis, SRL Diagnostics, Takeda, Eisai, Bristol‐Myers Squibb, EP‐CRSU, Pfizer, CMIC, Kobayashi Pharmaceutical, Shionogi, Astellas and Ascent Development Services; and took on a consulting or advisory roles for Eli Lilly and Ono.
Figures

References
-
- Kunimasa K., Matsumoto S., Kawamura T., et al., “Clinical Application of the AMOY 9‐In‐1 Panel to Lung Cancer Patients,” Lung Cancer 179 (2023): 107190. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

