A first-in-human, randomized study of the safety, pharmacokinetics and pharmacodynamics of povetacicept, an enhanced dual BAFF/APRIL antagonist, in healthy adults
- PMID: 39494621
- PMCID: PMC11532938
- DOI: 10.1111/cts.70055
A first-in-human, randomized study of the safety, pharmacokinetics and pharmacodynamics of povetacicept, an enhanced dual BAFF/APRIL antagonist, in healthy adults
Abstract
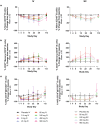
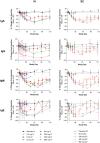
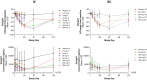
Therapeutic agents targeting the tumor necrosis factor (TNF) superfamily cytokines B-cell activating factor (BAFF, BLyS) and/or A PRoliferation Inducing Ligand (APRIL) have demonstrated clinical effectiveness in multiple autoimmune diseases, such as systemic lupus erythematosus, lupus nephritis, and immunoglobulin A nephropathy (IgAN). However, their clinical utility can often be limited by incomplete and/or prolonged times to clinical response and inconvenient dosing regimens, which may be improved by more potent dual inhibition of both cytokines. Povetacicept (ALPN-303; TACI vTD-Fc) is a crystallizable fragment (Fc) fusion protein of an engineered transmembrane activator and CAML interactor (TACI) domain which mediates more potent inhibitory activity than wild-type TACI-Fc or BAFF- or APRIL-specific antibodies and demonstrates superior pharmacokinetic and pharmacodynamic activity in multiple preclinical disease models. In this first-in-human study in healthy adults, povetacicept was well-tolerated as single ascending doses of up to 960 mg administered intravenously or subcutaneously. Dose-dependent pharmacokinetics were observed. Coverage of BAFF and APRIL was observed for 2-3 weeks and ≥4 weeks after doses of 80 mg and ≥240 mg, respectively. Maximal pharmacodynamic effects were observed at dose levels ≥80 mg for a single dose, associated with on-target reductions in antibody-secreting cells as well as in all circulating immunoglobulin isotypes, including the IgAN disease-related biomarker galactose-deficient-immunoglobulin A1 (Gd-IgA1), and were superior to results reported for wild-type TACI-Fc. These data strongly support further development of povetacicept for the treatment of B-cell-mediated automimmune diseases.
© 2024 Alpine Immune Sciences, a Vertex Company. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Rupert Davies, Stanford L. Peng, Amanda Enstrom, Allison G. Chunyk, Lori Blanchfield, NingXin Wang, Tiffany Blair, Heather M. Thomas, Alina Smith and Stacey R. Dillon are/were employees, shareholders, and/or officers (SLP) of Alpine Immune Sciences, Inc. Drs. Jason Lickliter and Kristi McLendon are employees of Nucleus Network.
Figures



References
-
- Samy E, Wax S, Huard B, Hess H, Schneider P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody‐associated diseases. Int Rev Immunol. 2017;36(1):3‐19. - PubMed
-
- Xu ZZ, Zhao BB, Xiong H, Wei BW, Wang YF. Serum BAFF and APRIL levels in patients with autoimmune hemolytic anemia and their clinical significance. Int J Hematol. 2015;102(4):394‐400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

