Preclinical Characterization of XB002, an Anti-Tissue Factor Antibody-Drug Conjugate for the Treatment of Solid Tumors
- PMID: 39494690
- PMCID: PMC11791478
- DOI: 10.1158/1535-7163.MCT-24-0002
Preclinical Characterization of XB002, an Anti-Tissue Factor Antibody-Drug Conjugate for the Treatment of Solid Tumors
Abstract
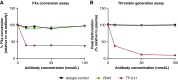
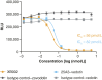
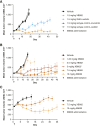
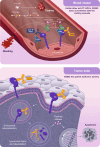
Tissue factor (TF) is overexpressed in various cancers and is typically associated with poor clinical outcomes. XB002 is an anti-TF antibody-drug conjugate designed to selectively deliver a cytotoxic payload to TF-expressing tumors while minimizing TF-related adverse events, particularly bleeding. The conjugate consists of a zovodotin linker-payload attached to a mAb (clone 25A3) that binds TF with high affinity (KD = 0.86 nmol/L). In vitro coagulation assays confirmed that 25A3 does not interfere with the clotting cascade; even at a concentration of 100 nmol/L, it did not affect the activation of coagulation factor X or thrombin generation. XB002 demonstrated efficient internalization in TF-expressing cancer cell lines, exhibiting potent cytotoxicity at subnanomolar concentrations. In the HPAF-II xenograft model, a regimen of XB002 (1.5 mg/kg, i.v.) administered once weekly for two weeks achieved complete tumor regression, with no detectable tumor growth up to five weeks after the second dose. In murine patient-derived xenograft models, a single dose of XB002 (10 mg/kg, i.v.) inhibited tumor growth across multiple cancer models, including bladder, cervical, gastric, head and neck squamous cell carcinoma, and non-small cell lung cancers. Remarkably, complete tumor regression was observed in the cervical cancer and head and neck squamous cell carcinoma models within 30 days of treatment. In nonhuman primate studies, XB002 demonstrated favorable pharmacokinetics with exposure in the desired therapeutic range and no signs of bleeding or neutropenia. Collectively, these data highlight XB002's broad-spectrum antitumor activity and strongly support its further clinical development.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S. Kantak reports other support from Exelixis, Inc. during the conduct of the study. R. Faggioni was an employee and has an XB002-related patent pending at Exelixis, Inc. A.G. Cai reports a patent for WO/2017/181145 pending, a patent for WO/2019/136309 pending, and a patent for WO/2021/003399 pending. M.M. Bhatti reports other support from Atreca, Inc. and Soleil Labs, LLC during the conduct of the study; other support from GSK plc and Synthekine, Inc. outside the submitted work; and a patent for EphA2 Antibodies pending. J. Li reports other support from Exelixis, Inc. during the conduct of the study. I. Vainshtein reports other support from Exelixis, Inc. during the conduct of the study, as well as other support from AstraZeneca outside the submitted work. B.A. Mendelsohn reports other support from Exelixis, Inc. during the conduct of the study. J. Gaudreault reports personal fees from JJG Pharma (consulting) during the conduct of the study. No disclosures were reported by the other authors.
Figures


References
-
- Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018;38:709–25. - PubMed
-
- van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 2012;119:924–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

