Pegylated liposomal doxorubicin in partially platinum-sensitive, platinum-resistant, or platinum-refractory ovarian cancer: a prospective study
- PMID: 39494888
- PMCID: PMC11783305
- DOI: 10.1093/oncolo/oyae194
Pegylated liposomal doxorubicin in partially platinum-sensitive, platinum-resistant, or platinum-refractory ovarian cancer: a prospective study
Abstract
Background: This study aimed to evaluate the efficacy and safety of pegylated liposomal doxorubicin (PLD) for patients with partially platinum-sensitive, platinum-resistant, or platinum-refractory ovarian cancer.
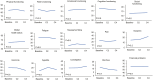
Methods: Patients with partially platinum-sensitive, platinum-resistant, or platinum-refractory ovarian cancer were recruited in this prospective, open-label, single-arm, multicenter study. Eligible patients were given 4-6 cycles of PLD (40 mg/m2 on day 1, every 4 weeks). The primary endpoint was progression-free survival (PFS). Secondary endpoints were overall survival (OS), objective response rate (ORR), disease control rate (DCR), quality of life, and safety. Exploratory endpoints included the change trend of CA125 and platinum-free interval.
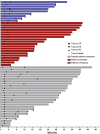
Results: Between June 2017 and November 2020, 167 eligible patients were included in the full analysis set. The median PFS and OS were 6.8 months (95% CI, 4.4-9.3 months) and 19.1 months (95% CI, 15.0-23.3 months), respectively. The ORR and DCR were 32.3% and 60.5%, respectively. The ORR (62.3 vs 22.5%) and DCR (84.9 vs 60.7%) of patients with a CA125 decrease after the first cycle were significantly higher than those without a CA125 decrease (all P < .05). Grade ≥ 3 and serious adverse events were reported in 9.9% and 3.9% of patients, respectively. No treatment-related death was observed.
Conclusion: PLD showed promising efficacy and manageable tolerability in patients with partially platinum-sensitive, platinum-resistant, or platinum-refractory ovarian cancer.ClinicalTrials.gov Identifier: Chinese Clinical Trial Registry, ChiCTR1900022962.
Keywords: ovarian cancer; partially platinum-sensitive; pegylated liposomal doxorubicin; platinum-refractory; platinum-resistant.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures



References
-
- Gordon AN, Tonda M, Sun S, Rackoff W; Doxil Study 30-49 Investigators. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95(1):1-8. 10.1016/j.ygyno.2004.07.011 - DOI - PubMed
-
- Cohn DE, Sill MW, Walker JL, et al. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: an NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2017;146(3):477-483. 10.1016/j.ygyno.2017.07.135 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

