The haemophilia joint health score for the assessment of joint health in patients with haemophilia
- PMID: 39494972
- PMCID: PMC11659503
- DOI: 10.1111/hae.15116
The haemophilia joint health score for the assessment of joint health in patients with haemophilia
Abstract
Introduction: The haemophilia joint health score (HJHS) is a tool used to assess joint changes in patients with haemophilia. There is lack of consensus on the interpretation of HJHS scores and their clinical relevance.
Aim: To evaluate available literature reporting HJHS changes over time and assess a possible cut-off value for clinically relevant outcomes and the ideal follow-up for a meaningful score change.
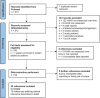
Methods: We conducted a literature search of studies published between 2011 and 2023 where the HJHS version 2.1 had been adopted to detect changes in joint health in patients with haemophilia. We focused on studies that assessed clinical relevance of HJHS changes, evaluated the use of cut-off values and reported a follow-up over time.
Results: Our search identified 213 publications of which 53 (25%) were deemed relevant for this review. Of these, 33 (62%) publications reported the total HJHS score and 20 (38%) reported a single joint HJHS score, while the way of reporting HJHS scores/change was highly variable. Ten publications (19%) assessed clinical relevance, but their methods of calculation differed (defining a cut-off score, measuring standardised response mean or minimal detectable change). The follow-up duration varied from 2 weeks to 8 years in these 10 studies.
Conclusions: High variability in assessing HJHS change over time is the primary consequence of its low sensitivity, and the lack of consensus on interpretation and clinical relevance of the score. Therefore, more sensitive tools should be used alongside HJHS to better define the joint health status of patients with haemophilia.
Keywords: clinical relevance; haemophilia; health; joint disease; joints.
© 2024 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
Cihan Ay received personal fees for lectures and/or participation in advisory boards from Bayer, Biomarin, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, LFB, Takeda, and SOBI. Maria Elisa Mancuso has acted as paid consultant, speaker and or advisor for Bayer, Biomarin, CSL Behring, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, and Takeda. Davide Matino reports research grants paid directly to the Institution (McMaster University) from Bayer, Pfizer, Novo Nordisk, Sanofi, Spark, Octapharma, Roche; personal fees/honoraria from Sanofi, Sobi, Novo Nordisk, Bayer, Pfizer, Octapharma, and Roche for participation in advisory boards, lectures and preparation of educational material. Karen Strike received research support from Hamilton Health Sciences Health Professional Clinical Research Award, Health Professional Investigator Award, McMaster Children's Hospital Foundation, Pfizer Canada, and Bayer; travel support from Pfizer, Bayer, Novo Nordisk; and consultation fees from Sanofi, Bayer, Hemalytic, Takeda, Pfizer, Biogen, Novo Nordisk, Roche, Baxalta, and Octapharma. Gianluigi Pasta received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or consulting from Bayer, Kedrion, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda.
Figures
References
-
- Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the International Hemophilia Prophylaxis Study Group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken). 2011;63(2):223‐230. - PubMed
-
- Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12(5):518‐525. - PubMed
-
- Feldman BM, Funk S, Hilliard P, et al. Hemophilia Joint Health Score (HJHS) 2.1. 2011; Accessed 15 January, 2024. https://elearning.wfh.org/resource/hemophilia‐joint‐health‐score‐hjhs/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical