ISG15 Drives Immune Pathology and Respiratory Failure during Systemic Lymphocytic Choriomeningitis Virus Infection
- PMID: 39495004
- PMCID: PMC11784630
- DOI: 10.4049/jimmunol.2400042
ISG15 Drives Immune Pathology and Respiratory Failure during Systemic Lymphocytic Choriomeningitis Virus Infection
Abstract
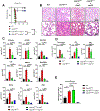
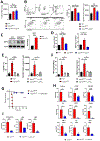
ISG15, an IFN-stimulated gene, plays a crucial role in modulating immune responses during viral infections. Its upregulation is part of the host's defense mechanism against viruses, contributing to the antiviral state of cells. However, altered ISG15 expression can also lead to immune dysregulation and pathological outcomes, particularly during persistent viral infections. Understanding the balance of ISG15 in promoting antiviral immunity while avoiding immune-mediated pathology is essential for developing targeted therapeutic interventions against viral diseases. In this article, using Usp18-deficient, USP18 enzymatic-inactive and Isg15-deficient mouse models, we report that a lack of USP18 enzymatic function during persistent viral infection leads to severe immune pathology characterized by hematological disruptions described by reductions in platelets, total WBCs, and lymphocyte counts; pulmonary cytokine amplification; lung vascular leakage; and death. The lack of Usp18 in myeloid cells mimicked the pathological manifestations observed in Usp18-/- mice and required Isg15. Mechanistically, interrupting the enzymes that conjugate/deconjugate ISG15, using Uba7-/- or Usp18C61A mice, respectively, led to accumulation of ISG15 that was accompanied by inflammatory neutrophil accumulation, lung pathology, and death similar to that observed in Usp18-deficient mice. Moreover, myeloid cell depletion reversed pathological manifestations, morbidity, and mortality in Usp18C61A mice. Our results suggest that dysregulated ISG15 production and signaling during persistent lymphocytic choriomeningitis virus infection can produce lethal immune pathology and could serve as a therapeutic target during severe viral infections with pulmonary pathological manifestations.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- R01 AI118862/AI/NIAID NIH HHS/United States
- SH 1140/2-1/Deutsche Forschungsgemeinschaft (DFG)
- R01 HL088256/HL/NHLBI NIH HHS/United States
- R01 CA232147/CA/NCI NIH HHS/United States
- P01 HL152958/HL/NHLBI NIH HHS/United States
- R01CA232147/Foundation for the National Institutes of Health (FNIH)
- R01 AI123210/AI/NIAID NIH HHS/United States
- R01 AI164744/AI/NIAID NIH HHS/United States
- AI123210/Foundation for the National Institutes of Health (FNIH)
- R01 CA177305/CA/NCI NIH HHS/United States
- FG KN 590/7-1/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Miscellaneous

