Enzyme Repertoires and Genomic Insights into Lycium barbarum Pectin Polysaccharide Biosynthesis
- PMID: 39495135
- PMCID: PMC12011363
- DOI: 10.1093/gpbjnl/qzae079
Enzyme Repertoires and Genomic Insights into Lycium barbarum Pectin Polysaccharide Biosynthesis
Abstract
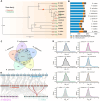
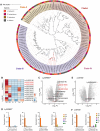
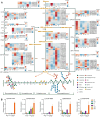
Lycium barbarum, a member of the Solanaceae family, is an important eudicot with applications in both food and medicine. L. barbarum pectin polysaccharides (LBPPs) are key bioactive compounds of L. barbarum, notable for being among the few polysaccharides with both biocompatibility and biomedical activity. Although studies have analyzed the functional properties of LBPPs, the mechanisms underlying their biosynthesis and transport by key enzymes remain poorly understood. In this study, we assembled a 2.18-Gb reference genome of L. barbarum, reconstructed the first complete biosynthesis pathway of LBPPs, and elucidated the sugar transport system. We also characterized the important genes responsible for backbone extension, sidechain synthesis, and modification of LBPPs. Furthermore, we characterized the long non-coding RNAs (lncRNAs) associated with polysaccharide metabolism. We identified a specific rhamnogalacturonan I (RG-I) rhamnosyltransferase, RRT3020, which enhances RG-I biosynthesis within LBPPs. These newly identified enzymes and pivotal genes endow L. barbarum with unique pectin biosynthesis capabilities, distinguishing it from other Solanaceae species. Our findings thus provide a foundation for evolutionary studies and molecular breeding to expand the diverse applications of L. barbarum.
Keywords: Lycium barbarum; Lycium barbarum pectin polysaccharide; Phylogenetic expansion; Rhamnogalacturonan I rhamnosyltransferase; lncRNA.
© The Author(s) 2024. Published by Oxford University Press and Science Press on behalf of the Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Conflict of interest statement
The authors declared no competing interests.
Figures




References
-
- Sun C, Chen X, Yang S, Jin C, Ding K, Chen C. LBP1C-2 from Lycium barbarum alleviated age-related bone loss by targeting BMPRIA/BMPRII/Noggin. Carbohydr Polym 2023;310:120725. - PubMed
-
- Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med 2010;76:7–19. - PubMed
-
- Tang L, Bao S, Du Y, Jiang Z, Wuliji AO, Ren X, et al. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed Pharmacother 2018;103:829–37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

