How well do different COVID-19 vaccines protect against different viral variants? A systematic review and meta-analysis
- PMID: 39495246
- PMCID: PMC11697103
- DOI: 10.1093/trstmh/trae082
How well do different COVID-19 vaccines protect against different viral variants? A systematic review and meta-analysis
Abstract
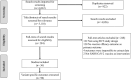
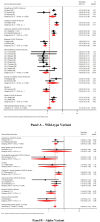
While the efficacy of coronavirus disease 2019 (COVID-19) vaccines has been evaluated in numerous trials, comprehensive evidence on how protection by different vaccines has varied over time remains limited. We aimed to compare protective effects of different vaccines against different viral variants. To achieve this, we searched Medline, Cochrane Library and Embase for randomized controlled trials assessing the efficacy of COVID-19 vaccines. Forest plots using Mantel-Haenszel and random-effects models were generated showing risk ratios (RRs) and 95% CIs by vaccines and variants. We included 36 studies with 90 variant-specific primary outcomes. We found a RR of 0.26 (95% CI 0.21 to 0.31) against all variants overall, with the highest protective effects against the wild-type (RR 0.13; 95% CI 0.10 to 0.18), followed by Alpha (RR 0.26; 95% CI 0.18 to 0.36), Gamma (RR 0.34; 95% CI 0.21 to 0.55), Delta (RR 0.39; 95% CI 0.28 to 0.56) and Beta (RR 0.49; 95% CI 0.40 to 0.62) variants. Nucleic acid vaccines showed the highest protection levels against all variants (RR 0.11; 95% CI 0.08 to 0.15), followed by protein subunit, inactivated virus and viral vector. In conclusion, we found high but heterogenous levels of protection for most COVID-19 vaccines, with decreasing protective effects for vaccines based on traditional technologies as SARS-CoV-2 variants emerged over time. Novel nucleic acid-based vaccines offered substantially higher and more consistent protection.
Keywords: COVID-19 vaccine efficacy; meta-analysis; systematic review; viral variants.
© The Author(s) 2024. Published by Oxford University Press on behalf of Royal Society of Tropical Medicine and Hygiene.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures




References
-
- World Health Organization . Statement for healthcare professionals: how COVID-19 vaccines are regulated for safety and effectiveness (Revised March 2022). WHO [accessed 22 March 2024].
-
- World Health Organization . The impact of COVID-19 on global health goals. WHO. Available at: https://www.who.int/news-room/spotlight/the-impact-of-covid-19-on-global... (2021).
-
- WHO . Tracking SARS-CoV-2 variants. WHO. (2023). Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [accessed 22 March 2024].
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

