Branched-chain amino acids and their metabolites decrease human and rat hepatic stellate cell activation
- PMID: 39495311
- PMCID: PMC11534903
- DOI: 10.1007/s11033-024-10027-4
Branched-chain amino acids and their metabolites decrease human and rat hepatic stellate cell activation
Abstract
Background: End-stage liver diseases (ESLDs) are a significant global health challenge due to their high prevalence and severe health impacts. Despite the severe outcomes associated with ESLDs, therapeutic options remain limited. Targeting the activation of hepatic stellate cells (HSCs), key drivers of extracellular matrix accumulation during liver injury presents a novel therapeutic approach. In ESLDs patients, branched-chain amino acids (BCAAs, leucine, isoleucine and valine) levels are decreased, and supplementation has been proposed to attenuate liver fibrosis and improve regeneration. However, their effects on HSCs require further investigation.
Objective: To evaluate the efficacy of BCAAs and their metabolites, branched-chain α-keto acids (BCKAs), in modulating HSCs activation in human and rat models.
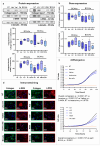
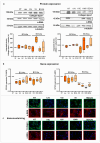
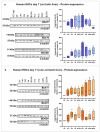
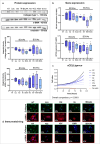
Methods: Primary HSCs from rats and cirrhotic and non-cirrhotic human livers, were cultured and treated with BCAAs or BCKAs to assess their effects on both preventing (from day 1 of isolation) and reversing (from day 7 of isolation) HSCs activation.
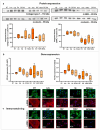
Results: In rat HSCs, leucine and BCKAs significantly reduced fibrotic markers and cell proliferation. In human HSCs, the metabolite of isoleucine decreased cell proliferation around 85% and increased the expression of branched-chain ketoacid dehydrogenase. The other metabolites also showed antifibrotic effects in HSCs from non-cirrhotic human livers.
Conclusion: BCAAs and their respective metabolites inhibit HSC activation with species-specific responses. Further research is needed to understand how BCAAs influence liver fibrogenesis. BCKAs supplementation could be a strategic approach for managing ESLDs, considering the nutritional status and amino acid profiles of patients.
Keywords: BCAAs; BCKAs; Branched-chain amino acids; Branched-chain keto acids; End-stage liver disease; Fibrosis; Hepatic stellate cells; Liver cirrhosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Dimou A, Tsimihodimos V, Bairaktari E (2022) The critical role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in human pathophysiology. Int J Mol Sci. 10.3390/IJMS23074022 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

