American Society for Microbiology evidence-based laboratory medicine practice guidelines to reduce blood culture contamination rates: a systematic review and meta-analysis
- PMID: 39495314
- PMCID: PMC11629619
- DOI: 10.1128/cmr.00087-24
American Society for Microbiology evidence-based laboratory medicine practice guidelines to reduce blood culture contamination rates: a systematic review and meta-analysis
Abstract
SUMMARYBlood cultures (BCs) are one of the critical tests used to detect bloodstream infections. BC results are not 100% specific. Interpretation of BC results is often complicated by detecting microbial contamination rather than true infection. False positives due to blood culture contamination (BCC) vary from 1% to as high as >10% of all BC results. False-positive BC results may result in patients undergoing unnecessary antimicrobial treatments, increased healthcare costs, and delay in detecting the true cause of infection or other non-infectious illness. Previous guidelines from the Clinical and Laboratory Standards Institute, College of American Pathologists, and others, based on expert opinion and surveys, promoted a limit of ≤3% as acceptable for BCC rates. However, the data supporting such recommendations are controversial. A previous systematic review of BCC examined three practices for reducing BCC rates (venipuncture, phlebotomy teams, and pre-packaged kits). Subsequently, numerous studies on different practices including using diversion devices, disinfectants, and education/training to lower BCC have been published. The goal of the current guideline is to identify beneficial intervention strategies to reduce BCC rates, including devices, practices, and education/training by providers in collaboration with the laboratory. We performed a systematic review of the literature between 2017 and 2022 using numerous databases. Of the 11,319 unique records identified, 311 articles were sought for full-text review, of which 177 were reviewed; 126 of the full-text articles were excluded based on pre-defined inclusion and exclusion criteria. Data were extracted from a total of 49 articles included in the final analysis. An evidenced-based committee's expert panel reviewed all the references as mentioned in Data Collection and determined if the articles met the inclusion criteria. Data from extractions were captured within an extraction template in the US Agency for Healthcare Research and Quality's Systematic Review Data Repository (https://srdr.ahrq.gov/). BCC rates were captured as the number of events (contaminated samples) per arm (standard practice versus improvement practice). Modified versions of the National Heart, Lung, and Blood Institute Study Quality Assessment Tools were used for risk of bias assessment (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). We used Grading of Recommendations, Assessment, Development and Evaluations to assess strength of evidence. There are several interventions that resulted in significant reduction in BCC rates: chlorhexidine as a disinfectant for skin preparation, using a diversion device prior to drawing BCs, using sterile technique practices, using a phlebotomy team to obtain BCs, and education/training programs. While there were no substantial differences between methods of decreasing BCC, our results indicate that the method of implementation can determine the success or failure of the intervention. Our evidence-based systematic review and meta-analysis support several interventions to effectively reduce BCC by approximately 40%-60%. However, devices alone without an education/training component and buy-in from key stakeholders to implement various interventions would not be as effective in reducing BCC rates.
Keywords: bacteremia; blood culture contamination; blood cultures; bloodstream infections; practice guidelines; systematic reviews.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



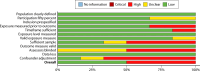









References
-
- Spellberg B. 2020. Edited by Bennett JE, Dolin RBlaseMartin J. Douglas, and Bennett’s principles and practice of infectious diseases. 9th ed, p 211–220. Elsevier, Philadelphia, PA.
-
- Doern GV, Carroll KC, Diekema DJ, Garey KW, Rupp ME, Weinstein MP, Sexton DJ. 2019. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev 33:00009–00019. doi:10.1128/CMR.00009-19 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

