Deferiprone in Alzheimer Disease: A Randomized Clinical Trial
- PMID: 39495531
- PMCID: PMC11536302
- DOI: 10.1001/jamaneurol.2024.3733
Deferiprone in Alzheimer Disease: A Randomized Clinical Trial
Abstract
Importance: Interventions that substantially slow neurodegeneration are needed to address the growing burden of Alzheimer disease (AD) to societies worldwide. Elevated brain iron observed in AD has been associated with accelerated cognitive decline and may be a tractable drug target.
Objective: To investigate whether the brain-permeable iron chelator deferiprone slows cognitive decline in people with AD.
Design, setting, and participants: This phase 2, double-masked, placebo-controlled randomized clinical trial of 12-month duration was conducted at 9 sites in Australia between August 2, 2018, and April 1, 2023. Patients older than 54 years with amyloid-confirmed mild cognitive impairment or early AD (a Mini-Mental State Examination score of 20 or higher) were screened. Randomization was 2:1 and masked to participants and all study staff.
Interventions: Deferiprone 15 mg/kg twice a day or placebo administered orally for 12 months.
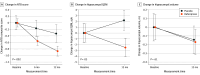
Main outcomes and measures: The primary outcome was a composite cognitive measure assessed at baseline, 6 months, and 12 months using a neuropsychological test battery (NTB) of memory, executive function, and attention tasks. Secondary outcomes included change in brain iron burden measured by quantitative susceptibility mapping (QSM) magnetic resonance imaging (target engagement), brain volume changes (secondary efficacy measure), and adverse events (safety analysis).
Results: Of 167 patients screened for eligibility, 81 were included, with 53 randomly assigned to the deferiprone group (mean [SD] age, 73.0 [8.0] years; 29 male [54.7%]) and 28 to the placebo group (mean [SD] age, 71.6 [7.2] years; 17 male [60.7%]); 54 participants completed the study (7 [25.0%] withdrew from the placebo group and 20 [37.7%] from the deferiprone group). In an intention-to-treat analysis, participants in the deferiprone group showed accelerated cognitive decline on the NTB primary outcome (β for interaction = -0.50; 95% CI, -0.80 to -0.20) compared with placebo (change in NTB composite z score for deferiprone, -0.80 [95% CI, -0.98 to -0.62]; for placebo, -0.30 [95% CI, -0.54 to -0.06]). Secondary analysis revealed that this result was driven by worsening performance on executive function tests. The QSM confirmed that deferiprone decreased iron in the hippocampus compared with placebo (change in hippocampal QSM for deferiprone, -0.36 ppb [95% CI, -0.76 to 0.04 ppb]; for placebo, 0.32 ppb [95% CI, -0.12 to 0.75 ppb]; β for interaction = -0.68 [95% CI, -1.27 to -0.09]). Longitudinal hippocampal volume loss was not affected by deferiprone, but exploratory analysis of other brain regions revealed increased volume loss with deferiprone in frontal areas. The frequency of the adverse effect of neutropenia (4 participants [7.5%] in the deferiprone group) was higher than in similar studies (1.6%-4.4%).
Conclusions: These trial findings show that deferiprone 15 mg/kg twice a day decreased hippocampal QSM and accelerated cognitive decline in patients with amyloid-confirmed early AD, suggesting that lowering iron with deferiprone is detrimental to patients with AD.
Trial registration: ClinicalTrials.gov Identifier: NCT03234686.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

