Characterization of Inflammatory Bowel Disease Heterogeneity Using Serum Proteomics: A Multicenter Study
- PMID: 39495605
- PMCID: PMC12063088
- DOI: 10.1093/ecco-jcc/jjae169
Characterization of Inflammatory Bowel Disease Heterogeneity Using Serum Proteomics: A Multicenter Study
Abstract
Background: Recent genetic and transcriptomic data highlight the need for improved molecular characterization of inflammatory bowel disease (IBD). Proteomics may advance the delineation of IBD phenotypes since it accounts for post-transcriptional modifications.
Aims: We aimed to assess the IBD spectrum based on inflammatory serum proteins and identify discriminative patterns of underlying biological subtypes across multiple European cohorts.
Methods: Using proximity extension methodology, we measured 86 inflammation-related serum proteins in 1551 IBD patients and 312 healthy controls (HC). We screened for proteins exhibiting significantly different levels among IBD subtypes and between IBD and HC. Classification models for differentiating between Crohn's disease (CD) and ulcerative colitis (UC) were employed to explore the IBD spectrum based on estimated probability scores.
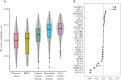
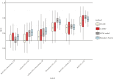
Results: Levels of multiple proteins, such as interleukin-17A, matrix metalloproteinase-10, and fibroblast growth factor-19, differed (fold-change >1.2; false discovery rate <0.05) between ileal versus colonic IBD. Using multivariable models, a protein signature reflecting the IBD spectrum was identified, positioning colonic CD between UC and ileal CD, which were at opposite ends of the spectrum. Based on area under the curve (AUC) estimates, classification models more accurately differentiated UC from ileal CD (median AUCs > 0.73) than colonic CD (median AUCs < 0.62). Models differentiating colonic CD from ileal CD demonstrated intermediate performance (median AUCs: 0.67-0.69).
Conclusions: Our findings in serum proteins support the presence of a continuous IBD spectrum rather than a clear separation of CD and UC. Within the spectrum, disease location may reflect a more similar disease than CD versus UC, as colonic CD resembled UC more closely than ileal CD.
Keywords: Crohn’s disease; Montreal classification; biomarkers; disease location; serum proteins; ulcerative colitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
JH served as a speaker and/or advisory board member for AbbVie, BMS, Celgene, Celltrion, Dr. Falk Pharma and the Falk Foundation, Ferring, Galapagos, Gilead, Hospira, Index Pharma, Janssen, MEDA, Medivir, MSD, Novartis, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and UCB, and received grant support from Janssen, MSD, and Takeda. SV reports research support from AbbVie, Johnson & Johnson, Pfizer, and Takeda; lecture fees from AbbVie, Centocor, Ferring, Genentech/Roche, Hospira, Johnson & Johnson, Merck Sharp & Dohme, Pfizer, Takeda, and Tillotts, and consulting fees from AbbVie, Abivax, Celgene, Celltrion, Centocor, Ferring, Galapagos, Genentech/Roche, Gilead, GlaxoSmithKline, Hospira, Johnson & Johnson, Merck Sharp & Dohme, Mundipharma, Pfizer, ProDigest, Prometheus, Second Genome, Takeda, and Tillotts. BV has received research support for research from Pfizer, speaker’s fees from Abbvie, Biogen, Bristol Myers Squibb, Chiesi, Falk, Ferring, Galapagos, Janssen, MSD, Pfizer, R-Biopharm, Takeda, Truvion and Viatris and consultancy fees from Abbvie, Alimentiv, Applied Strategic, Atheneum, Bristol Myers Squibb, Galapagos, Guidepont, Ipsos, Janssen, Progenity, Sandoz, Sosei Heptares, Takeda, Tillots Pharma, and Viatris. DB served as a speaker and/or advisory board member for BMS, Janssen, Pfizer, Pharmacosmos, Sandoz, Takeda, and Tillotts Pharma. CRHH has served as a speaker and/or advisory board member for AstraZeneca, Dr Falk Pharma and the Falk Foundation, Galapagos, Janssen, Pfizer, Takeda, Tillotts Pharma, and received grant support from Tillotts and Takeda. MC has received speaker’s fees from ViforPharma. She is the national PI for clinical trials for AstraZeneca. None of these activities have any relation to the present study. LÖ has received financial support for research by Genetic Analysis AS, Biocodex, Danone Research, and AstraZeneca, and served as Consultant/Advisory Board member for Genetic Analysis AS, and as a speaker for Biocodex, Ferring Pharmaceuticals, Takeda, AbbVie, Novartis, Janssen-Cilag, and Meda. SA (included in the BIO IBD consortium) served as a member of Scientific committee/Advisory board of Pharmacosmos, Janssen, Takeda, as a consultant for Takeda, Janssen, and as a speaker: Galapagos, Janssen, Tillotts, received research grants from OrionPharma and Janssen. AC (included in the BIO IBD consortium) served as a speaker for Takeda and Tillotts Pharma. RU (included in the COLLIBRI consortium) served as a member of the Advisory Board and/or consulting for AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Lilly, Pfizer, Roivant, and Takeda, and funded by a K23 Career Development Award K23 KD111995-01A1. KA (included in the COLLIBRI consortium) served as a speaker and/or advisory board member for BMS, Janssen, Pfizer, Falk, Lilly, Galapagos, and Takeda Pharma. The other authors report no disclosures relevant to the manuscript.
Figures



Comment in
-
Decoding the proteomic landscape of inflammatory bowel disease.J Crohns Colitis. 2025 Jan 11;19(1):jjaf008. doi: 10.1093/ecco-jcc/jjaf008. J Crohns Colitis. 2025. PMID: 39891576 No abstract available.
References
-
- Dolinger M, Torres J, Vermeire S.. Crohn’s disease. Lancet 2024;403:1177–91. - PubMed
-
- Le Berre C, Honap S, Peyrin-Biroulet L.. Ulcerative colitis. Lancet 2023;402:571–84. - PubMed
-
- Maaser C, Sturm A, Vavricka SR, et al.; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. - PubMed
-
- Silverberg MS, Satsangi J, Ahmad T, et al.. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A–36A. - PubMed

