Exosomal miR-155-5p promote the occurrence of carotid atherosclerosis
- PMID: 39495676
- PMCID: PMC11534067
- DOI: 10.1111/jcmm.70187
Exosomal miR-155-5p promote the occurrence of carotid atherosclerosis
Abstract
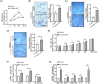
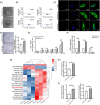
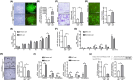
Periodontitis is a significant independent risk factor for atherosclerosis. Yet, the exact mechanism of action is still not fully understood. In this study, we investigated the effect of exosomes-miR-155-5p derived from periodontal endothelial cells on atherosclerosis in vitro and in vivo. Higher expression of miR-155-5p was detected in the plasma exosomes of patients with chronic periodontitis (CP) and carotid atherosclerosis (CAS) compared to patients with CP. Also, the expression level of miR-155-5p was associated with the severity of CP. miR-155-5p-enriched exosomes from HUVECs increased the angiogenesis and permeability of HAECs and promoted the expression of angiogenesis, permeability, and inflammation genes. Along with the overexpression or inhibition of miR-155-5p, the biological effect of HUVECs-derived exosomes on HAECs changed correspondingly. In ApoE-/- mouse models, miR-155-5p-enriched exosomes promoted the occurrence of carotid atherosclerosis by increasing permeable and angiogenic activity. Collectively, these findings highlight a molecular mechanism of periodontitis in CAS, uncovering exosomal miR-155-5p derived periodontitis affecting carotid endothelial cells in an 'exosomecrine' manner. Exosomal miR-155-5p may be used as a biomarker and target for clinical intervention to control this intractable disease in future, and the graphic abstract was shown in Figure S1.
Keywords: LPS; carotid atherosclerosis; chronic periodontitis; exosome; miR‐155‐5p.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559‐572. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

