Disentangling Neurodegeneration From Aging in Multiple Sclerosis Using Deep Learning: The Brain-Predicted Disease Duration Gap
- PMID: 39496109
- PMCID: PMC11540460
- DOI: 10.1212/WNL.0000000000209976
Disentangling Neurodegeneration From Aging in Multiple Sclerosis Using Deep Learning: The Brain-Predicted Disease Duration Gap
Abstract
Background and objectives: Disentangling brain aging from disease-related neurodegeneration in patients with multiple sclerosis (PwMS) is increasingly topical. The brain-age paradigm offers a window into this problem but may miss disease-specific effects. In this study, we investigated whether a disease-specific model might complement the brain-age gap (BAG) by capturing aspects unique to MS.
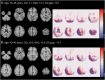
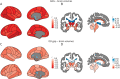
Methods: In this retrospective study, we collected 3D T1-weighted brain MRI scans of PwMS to build (1) a cross-sectional multicentric cohort for age and disease duration (DD) modeling and (2) a longitudinal single-center cohort of patients with early MS as a clinical use case. We trained and evaluated a 3D DenseNet architecture to predict DD from minimally preprocessed images while age predictions were obtained with the DeepBrainNet model. The brain-predicted DD gap (the difference between predicted and actual duration) was proposed as a DD-adjusted global measure of MS-specific brain damage. Model predictions were scrutinized to assess the influence of lesions and brain volumes while the DD gap was biologically and clinically validated within a linear model framework assessing its relationship with BAG and physical disability measured with the Expanded Disability Status Scale (EDSS).
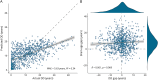
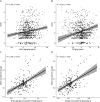
Results: We gathered MRI scans of 4,392 PwMS (69.7% female, age: 42.8 ± 10.6 years, DD: 11.4 ± 9.3 years) from 15 centers while the early MS cohort included 749 sessions from 252 patients (64.7% female, age: 34.5 ± 8.3 years, DD: 0.7 ± 1.2 years). Our model predicted DD better than chance (mean absolute error = 5.63 years, R2 = 0.34) and was nearly orthogonal to the brain-age model (correlation between DD and BAGs: r = 0.06 [0.00-0.13], p = 0.07). Predictions were influenced by distributed variations in brain volume and, unlike brain-predicted age, were sensitive to MS lesions (difference between unfilled and filled scans: 0.55 years [0.51-0.59], p < 0.001). DD gap significantly explained EDSS changes (B = 0.060 [0.038-0.082], p < 0.001), adding to BAG (ΔR2 = 0.012, p < 0.001). Longitudinally, increasing DD gap was associated with greater annualized EDSS change (r = 0.50 [0.39-0.60], p < 0.001), with an incremental contribution in explaining disability worsening compared with changes in BAG alone (ΔR2 = 0.064, p < 0.001).
Discussion: The brain-predicted DD gap is sensitive to MS-related lesions and brain atrophy, adds to the brain-age paradigm in explaining physical disability both cross-sectionally and longitudinally, and may be used as an MS-specific biomarker of disease severity and progression.
Conflict of interest statement
G. Pontillo received research grants from ECTRIMS-MAGNIMS and ESNR. M. Calabrese received speaker honoraria from Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, and Roche, and receives research support from the Progressive MS Alliance and Italian Minister of Health. S. Cocozza discloses honoraria for advisory board participation from Amicus and research grants from Fondazione Italiana Sclerosi Multipla, and Telethon. R. Cortese received speaker honoraria and travel support from Roche, Merck, Sanofi-Genzyme, Novartis, and Janssen, and was awarded a MAGNIMS-ECTRIMS fellowship in 2019. M. Filippi is the Editor-in-Chief of the
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
