Developing a Core Outcome Set for Netherton Syndrome: An International Multi-Stakeholder e-Delphi Consensus Study
- PMID: 39496226
- PMCID: PMC11793099
- DOI: 10.1159/000542215
Developing a Core Outcome Set for Netherton Syndrome: An International Multi-Stakeholder e-Delphi Consensus Study
Abstract
Introduction: Netherton syndrome (NS; OMIM#256500) is a rare and severe disorder of epidermal maturation and keratinization caused by pathogenic variants in the serine protease inhibitor Kazal type 5 (SPINK5), leading to severe skin barrier impairment. Although effective treatment is crucial for NS patients, there is a lack of knowledge on what the best treatment options are for these patients. Large heterogeneity in reported outcomes and measurement instruments hinders accurate comparison of treatment results across studies and the development of a treatment guideline. Therefore, we aimed to develop a core outcome set (COS) for NS that can be used in clinical care and research.
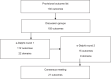
Methods: This study was performed in accordance with the recommendations of the Core Outcome Measures in Effectiveness Trials (COMET) initiative. After identification of outcomes through a literature search and classification based on the International Classification of Functioning and taxonomies published by the COMET initiative, discussion groups were organized at the 2nd International Netherton Congress 2022 to finalize the provisional outcome list. Through a 2-round e-Delphi, 41 stakeholders (patients and family members, professionals, and representatives of industry) from 14 countries rated the importance of the outcomes using a 9-point Likert scale. An online consensus meeting attended by 14 stakeholders finalized the COS.
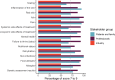
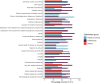
Results: The COS for NS comprised 21 outcomes in 10 domains. These included four "skin" outcomes, two "sensation" outcomes, two "side-effects of treatment" outcomes, one "vitality" outcome, one "emotional functioning" outcome, two "physical development" outcomes, two "nutrition" outcomes, two "infections" outcomes, two "allergies" outcomes, and three "assessment results" outcomes.
Conclusion: In this study, consensus was reached on 21 outcomes to be included in the COS for NS. The selection of outcomes in the COS underlines that NS not only affects the skin but is a disease requiring a broad multidisciplinary approach in clinical care and research. International implementation of this COS will lead to more uniform reporting, thereby enabling comparison of study results, which may facilitate future treatment guideline development. The next step is to further conceptually define the outcomes and reach consensus on how to measure these.
Introduction: Netherton syndrome (NS; OMIM#256500) is a rare and severe disorder of epidermal maturation and keratinization caused by pathogenic variants in the serine protease inhibitor Kazal type 5 (SPINK5), leading to severe skin barrier impairment. Although effective treatment is crucial for NS patients, there is a lack of knowledge on what the best treatment options are for these patients. Large heterogeneity in reported outcomes and measurement instruments hinders accurate comparison of treatment results across studies and the development of a treatment guideline. Therefore, we aimed to develop a core outcome set (COS) for NS that can be used in clinical care and research.
Methods: This study was performed in accordance with the recommendations of the Core Outcome Measures in Effectiveness Trials (COMET) initiative. After identification of outcomes through a literature search and classification based on the International Classification of Functioning and taxonomies published by the COMET initiative, discussion groups were organized at the 2nd International Netherton Congress 2022 to finalize the provisional outcome list. Through a 2-round e-Delphi, 41 stakeholders (patients and family members, professionals, and representatives of industry) from 14 countries rated the importance of the outcomes using a 9-point Likert scale. An online consensus meeting attended by 14 stakeholders finalized the COS.
Results: The COS for NS comprised 21 outcomes in 10 domains. These included four "skin" outcomes, two "sensation" outcomes, two "side-effects of treatment" outcomes, one "vitality" outcome, one "emotional functioning" outcome, two "physical development" outcomes, two "nutrition" outcomes, two "infections" outcomes, two "allergies" outcomes, and three "assessment results" outcomes.
Conclusion: In this study, consensus was reached on 21 outcomes to be included in the COS for NS. The selection of outcomes in the COS underlines that NS not only affects the skin but is a disease requiring a broad multidisciplinary approach in clinical care and research. International implementation of this COS will lead to more uniform reporting, thereby enabling comparison of study results, which may facilitate future treatment guideline development. The next step is to further conceptually define the outcomes and reach consensus on how to measure these.
Keywords: Core outcome set; Netherton syndrome; Outcome; SPINK5; e-Delphi.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. . Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25(2):141–2. - PubMed
-
- Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013;351(2):289–300. - PubMed
-
- Sarri CA, Roussaki-Schulze A, Vasilopoulos Y, Zafiriou E, Patsatsi A, Stamatis C, et al. . Netherton syndrome: a genotype-phenotype review. Mol Diagn Ther. 2017;21(2):137–52. - PubMed
-
- Bitoun E, Micheloni A, Lamant L, Bonnart C, Tartaglia-Polcini A, Cobbold C, et al. . LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Hum Mol Genet. 2003;12(19):2417–30. - PubMed
-
- Schechter NM, Choi EJ, Wang ZM, Hanakawa Y, Stanley JR, Kang Y, et al. . Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI). Biol Chem. 2005;386(11):1173–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

