Human iPSC-Derived MSCs Induce Neurotrophic Effects and Improve Metabolic Activity in Acute Neuronal Injury Models
- PMID: 39496487
- PMCID: PMC11694398
- DOI: 10.1523/JNEUROSCI.0606-24.2024
Human iPSC-Derived MSCs Induce Neurotrophic Effects and Improve Metabolic Activity in Acute Neuronal Injury Models
Abstract
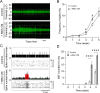
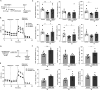
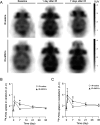
Mesenchymal stromal cell (MSC) therapy has regenerative potentials to treat various pathological conditions including neurological diseases. MSCs isolated from various organs can differentiate into specific cell types to repair organ damages. However, their paracrine mechanisms are predicted to predominantly mediate their immunomodulatory, proangiogenic, and regenerative properties. While preclinical studies highlight the significant potential of MSC therapy in mitigating neurological damage from stroke and traumatic brain injury, the variability in clinical trial outcomes may stem from the inherent heterogeneity of somatic MSCs. Accumulating evidence has demonstrated that induced pluripotent stem cells (iPSCs) are an ideal alternative resource for the unlimited expansion and biomanufacturing of MSCs. Thus, we investigated how iPSC-derived MSCs (iMSCs) influence properties of iPSC-derived neurons. Our findings demonstrate that the secretome from iMSCs possesses neurotrophic effects, improving neuronal survival and promoting neuronal outgrowth and synaptic activity in vitro. Additionally, the iMSCs enhance metabolic activity via mitochondrial respiration in neurons, both in vitro and in mouse models. Glycolytic pathways also increased following the administration of iMSC secretome to iPSC-derived neurons. Consistently, in vivo experiments showed that intravenous administration of iMSCs compensated for the elevated energetic demand in male mice with irradiation-induced brain injury by restoring synaptic metabolic activity during acute brain damage. 18F-FDG PET imaging also detected an increase in brain glucose uptake following iMSC administration. Together, our results highlight the potential of iMSC-based therapy in treating neuronal damage in various neurological disorders, while paving the way for future research and potential clinical applications of iMSCs in regenerative medicine.
Keywords: MSC; iPSC; neuron; neurotrophic effect; stem cell therapy.
Copyright © 2024 Kawatani et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
