Piezo1 facilitates the initiation and progression of renal fibrosis by mediating cell apoptosis and mitochondrial dysfunction
- PMID: 39496543
- PMCID: PMC11536639
- DOI: 10.1080/0886022X.2024.2415519
Piezo1 facilitates the initiation and progression of renal fibrosis by mediating cell apoptosis and mitochondrial dysfunction
Abstract
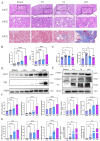
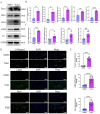
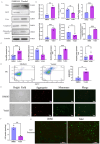
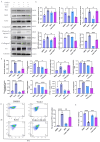
Renal fibrosis is the major pathological changes of Chronic kidney disease (CKD). Piezo1, a mechanical sensitive ion channel, is implicated in organ fibrosis. However, the precise role of Piezo1 in CKD fibrosis is unknown. The aims of this study were to identify that the role of Piezo1 in CKD fibrosis and its potential involvement of mitochondrial dysfunction. We performed the study with the Piezo1 agonist Yoda1, Bax inhibitor BAI1, Piezo1 inhibitor GsMTx4 and detected the injury, fibrosis, apoptosis markers and mitochondrial dysfunction. The results showed that the levels of apoptosis, mitochondrial dysfunction, injury and fibrosis increased in TCMK-1 cells after treatment with Yoda1. However, these changes that induced by Yoda1 were relieved by BAI1. Similarly, inhibition Piezo1 with GsMTx4 also partly relieved the renal injury, renal fibrosis, apoptosis and mitochondrial dysfunction in vivo and vitro. In conclusion, we found Piezo1 promoted the initiation and development of renal fibrosis and inhibiting Piezo1 improved the fibrosis.
Keywords: Chronic kidney disease; Piezo1; apoptosis; mitochondrial dysfunction; renal fibrosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





Similar articles
-
PIEZO1 targeting in macrophages boosts phagocytic activity and foam cell apoptosis in atherosclerosis.Cell Mol Life Sci. 2024 Aug 6;81(1):331. doi: 10.1007/s00018-024-05372-3. Cell Mol Life Sci. 2024. PMID: 39107572 Free PMC article.
-
Mechanosensitive Cation Channel Piezo1 Is Involved in Renal Fibrosis Induction.Int J Mol Sci. 2024 Jan 31;25(3):1718. doi: 10.3390/ijms25031718. Int J Mol Sci. 2024. PMID: 38338996 Free PMC article. Review.
-
Mechanosensitive Piezo1 channels mediate renal fibrosis.JCI Insight. 2022 Apr 8;7(7):e152330. doi: 10.1172/jci.insight.152330. JCI Insight. 2022. PMID: 35230979 Free PMC article.
-
Single-cell sequencing reveals the impact of endothelial cell PIEZO1 expression on thoracic aortic aneurysm.J Mol Cell Cardiol. 2024 Jun;191:63-75. doi: 10.1016/j.yjmcc.2024.04.015. Epub 2024 May 7. J Mol Cell Cardiol. 2024. PMID: 38718563
-
Mitochondrial dysfunction: the hidden catalyst in chronic kidney disease progression.Ren Fail. 2025 Dec;47(1):2506812. doi: 10.1080/0886022X.2025.2506812. Epub 2025 May 29. Ren Fail. 2025. PMID: 40441691 Free PMC article. Review.
Cited by
-
Targeting ion channel networks in diabetic kidney disease: from molecular crosstalk to precision therapeutics and clinical innovation.Front Med (Lausanne). 2025 Jun 26;12:1607701. doi: 10.3389/fmed.2025.1607701. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40641971 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
