Genome-guided isolation of the hyperthermophilic aerobe Fervidibacter sacchari reveals conserved polysaccharide metabolism in the Armatimonadota
- PMID: 39496591
- PMCID: PMC11535203
- DOI: 10.1038/s41467-024-53784-3
Genome-guided isolation of the hyperthermophilic aerobe Fervidibacter sacchari reveals conserved polysaccharide metabolism in the Armatimonadota
Erratum in
-
Author Correction: Genome-guided isolation of the hyperthermophilic aerobe Fervidibacter sacchari reveals conserved polysaccharide metabolism in the Armatimonadota.Nat Commun. 2025 Apr 29;16(1):4015. doi: 10.1038/s41467-025-59415-9. Nat Commun. 2025. PMID: 40301321 Free PMC article. No abstract available.
Abstract
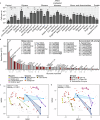
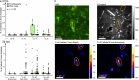
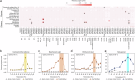
Few aerobic hyperthermophilic microorganisms degrade polysaccharides. Here, we describe the genome-enabled enrichment and optical tweezer-based isolation of an aerobic polysaccharide-degrading hyperthermophile, Fervidibacter sacchari, previously ascribed to candidate phylum Fervidibacteria. F. sacchari uses polysaccharides and monosaccharides for growth at 65-87.5 °C and expresses 191 carbohydrate-active enzymes (CAZymes) according to RNA-Seq and proteomics, including 31 with unusual glycoside hydrolase domains (GH109, GH177, GH179). Fluorescence in-situ hybridization and nanoscale secondary ion mass spectrometry confirmed rapid assimilation of 13C-starch in spring sediments. Purified GHs were optimally active at 80-100 °C on ten different polysaccharides. Finally, we propose reassigning Fervidibacteria as a class within phylum Armatimonadota, along with 18 other species, and show that a high number and diversity of CAZymes is a hallmark of the phylum, in both aerobic and anaerobic lineages. Our study establishes Fervidibacteria as hyperthermophilic polysaccharide degraders in terrestrial geothermal springs and suggests a broad role for Armatimonadota in polysaccharide catabolism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- BioProject/PRJNA1086027
- Actions
Grants and funding
- 91951205/National Natural Science Foundation of China (National Science Foundation of China)
- 80NNSC17KO548/National Aeronautics and Space Administration (NASA)
- 80NSSC20M0043/Nevada Space Grant Consortium (Nevada NASA Space Grant Consortium)
- P20 GM103440/GM/NIGMS NIH HHS/United States
- GM103440/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

