Nerve injury inhibits Oprd1 and Cnr1 transcription through REST in primary sensory neurons
- PMID: 39496614
- PMCID: PMC11535536
- DOI: 10.1038/s41598-024-74487-1
Nerve injury inhibits Oprd1 and Cnr1 transcription through REST in primary sensory neurons
Abstract
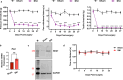
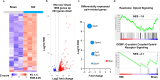
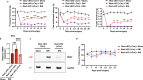
The transcription repressor REST in the dorsal root ganglion (DRG) is upregulated by peripheral nerve injury and promotes the development of chronic pain. However, the genes targeted by REST in neuropathic pain development remain unclear. The expression levels of four opioid receptor genes (Oprm1, Oprd1, Oprl1 and Oprk1) and the cannabinoid CB1 receptor (Cnr1) gene in the DRG regulate nociception. In this study, we determined the role of REST in controlling their expression in the DRG induced by spared nerve injury (SNI). SNI induced chronic pain hypersensitivity in wild-type mice and was accompanied by increased levels of Rest transcript and protein. Transcriptomic analyses of wild-type mouse DRGs suggested that SNI upregulates the expression of Rest transcripts and downregulates the transcripts of all four opioid receptor genes and the Cnr1 gene. Quantitative reverse transcription polymerase chain reaction analyses of these tissues validated these results. Analysis of publicly available bioinformatic data suggested that REST binds to the promoter regions of Oprm1 and Cnr1. Chromatin immunoprecipitation analyses indicated the presence of REST at these promoters. Full-length Rest conditional knockout in primary sensory neurons reduced SNI-induced pain hypersensitivity and rescued the SNI-induced reduction in the expression of Oprd1 and Cnr1 in mouse DRG. Our results suggest that nerve injury represses the transcription of at least the Oprd1 and Cnr1 genes via REST in primary sensory neurons and that REST is a potential therapeutic target for neuropathic pain. Thus, inhibiting REST activity could potentially reduce chronic neuropathic pain and augment opioid/cannabinoid analgesic actions by increasing the transcription of Oprd1 and Cnr1 genes in DRG neurons.
Keywords: Cannabinoid CB1 receptor; Chronic pain; Dorsal root ganglion; Opioid receptors; REST; Therapeutic target.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

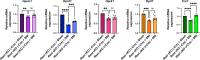
Update of
-
Nerve injury inhibits Oprd1 and Cnr1 transcription through REST in primary sensory neurons.bioRxiv [Preprint]. 2024 Apr 8:2024.02.17.579842. doi: 10.1101/2024.02.17.579842. bioRxiv. 2024. Update in: Sci Rep. 2024 Nov 4;14(1):26612. doi: 10.1038/s41598-024-74487-1. PMID: 38585789 Free PMC article. Updated. Preprint.
Similar articles
-
Nerve injury inhibits Oprd1 and Cnr1 transcription through REST in primary sensory neurons.bioRxiv [Preprint]. 2024 Apr 8:2024.02.17.579842. doi: 10.1101/2024.02.17.579842. bioRxiv. 2024. Update in: Sci Rep. 2024 Nov 4;14(1):26612. doi: 10.1038/s41598-024-74487-1. PMID: 38585789 Free PMC article. Updated. Preprint.
-
G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury.Mol Pain. 2016 Dec 7;12:1744806916682242. doi: 10.1177/1744806916682242. Print 2016. Mol Pain. 2016. PMID: 27927796 Free PMC article.
-
Nerve Injury Diminishes Opioid Analgesia through Lysine Methyltransferase-mediated Transcriptional Repression of μ-Opioid Receptors in Primary Sensory Neurons.J Biol Chem. 2016 Apr 15;291(16):8475-85. doi: 10.1074/jbc.M115.711812. Epub 2016 Feb 25. J Biol Chem. 2016. PMID: 26917724 Free PMC article.
-
Established and emerging roles of protein kinases in regulating primary sensory neurons in injury-and inflammation-associated pain.Expert Opin Ther Targets. 2025 Apr-May;29(4-5):267-280. doi: 10.1080/14728222.2025.2489540. Epub 2025 Apr 13. Expert Opin Ther Targets. 2025. PMID: 40200157 Review.
-
Characterization of an agonist probe for opioid receptor mu 1 (OPRM1)-opioid receptor delta 1 (OPRD1) heterodimerization.2012 Dec 17 [updated 2013 Apr 5]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2012 Dec 17 [updated 2013 Apr 5]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23833799 Free Books & Documents. Review.
Cited by
-
Gut sensory neurons as regulators of neuro-immune-microbial interactions: from molecular mechanisms to precision therapy for IBD/IBS.J Neuroinflammation. 2025 Jul 2;22(1):172. doi: 10.1186/s12974-025-03500-9. J Neuroinflammation. 2025. PMID: 40605050 Free PMC article. Review.
References
-
- Ghosh, K. & Pan, H. L. Epigenetic mechanisms of neural plasticity in Chronic Neuropathic Pain. ACS Chem. Neurosci.13 (4). 10.1021/acschemneuro.1c00841 (2022). - PubMed
-
- Thouaye, M. & Yalcin, I. Neuropathic pain: from actual pharmacological treatments to new therapeutic horizons. Pharmacol. Ther.25110.1016/j.pharmthera.2023.108546 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

