The Arabidopsis receptor-like kinase WAKL4 limits cadmium uptake via phosphorylation and degradation of NRAMP1 transporter
- PMID: 39496660
- PMCID: PMC11535502
- DOI: 10.1038/s41467-024-53898-8
The Arabidopsis receptor-like kinase WAKL4 limits cadmium uptake via phosphorylation and degradation of NRAMP1 transporter
Abstract
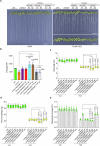
Cadmium (Cd) is a detrimental heavy metal propagated from soil to the food chain via plants, posing a great risk to human health upon consumption. Despite the understanding of Cd tolerance mechanisms in plants, whether and how plants actively respond to Cd and in turn restrict its uptake and accumulation remain elusive. Here, we identify a cell wall-associated receptor-like kinase 4 (WAKL4) involved in specific tolerance to Cd stress. We show that Cd rapidly and exclusively induces WAKL4 accumulation by promoting WAKL4 transcription and blocking its vacuole-dependent proteolysis in roots. The accumulated WAKL4 next interacts with and phosphorylates the Cd transporter NRAMP1 at Tyr488, leading to the enhanced ubiquitination and vacuole-dependent degradation of NRAMP1, and consequently reducing Cd uptake. Our findings therefore uncover a mechanism conferred by the WAKL4-NRAMP1 module that enables plants to actively respond to Cd and limit its uptake, informing the future molecular breeding of low Cd accumulated crops or vegetables.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Zhao, F. J., Tang, Z., Song, J. J., Huang, X. Y. & Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant15, 27–44 (2022). - PubMed
-
- Yan, H. L. et al. Cadmium contamination in food crops: Risk assessment and control in smart age. Crit. Rev. Env. Sci. Tec.53, 1643-1661 (2023).
-
- Huang, Y. et al. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct.8, 1373–1401 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

