Histidine containing dipeptides protect epithelial and endothelial cell barriers from methylglyoxal induced injury
- PMID: 39496731
- PMCID: PMC11535046
- DOI: 10.1038/s41598-024-77891-9
Histidine containing dipeptides protect epithelial and endothelial cell barriers from methylglyoxal induced injury
Abstract
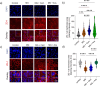
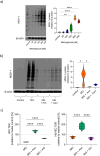
Integrity of epithelial and endothelial cell barriers is of critical importance for health, barrier disruption is a hallmark of numerous diseases, of which many are driven by carbonyl stressors such as methylglyoxal (MG). Carnosine and anserine exert some MG-quenching activity, but the impact of these and of other histidine containing dipeptides on cell barrier integrity has not been explored in detail. In human proximal tubular (HK-2) and umbilical vein endothelial (HUVEC) cells, exposure to 200 µM MG decreased transepithelial resistance (TER), i.e. increased ionic permeability and permeability for 4-, 10- and 70-kDa dextran, membrane zonula occludens (ZO-1) abundance was reduced, methylglyoxal 5-hydro-5-methylimidazolones (MG-H1) formation was increased. Carnosine, balenine (ß-ala-1methyl-histidine) and anserine (ß-ala-3-methyl-histidine) ameliorated MG-induced reduction of TER in both cell types. Incubation with histidine, 1-/3-methylhistidine, but not with ß-alanine alone, restored TER, although to a lower extent than the corresponding dipeptides. Carnosine and anserine normalized transport and membrane ZO-1 abundance. Aminoguanidine, a well-described MG-quencher, did not mitigate MG-induced loss of TER. Our results show that the effects of the dipeptides on epithelial and endothelial resistance and junction function depend on the methylation status of histidine and are not exclusively explained by their quenching activity.
Keywords: Barrier integrity; Carbonyl stress; Dipeptides; Ionic permeability; Transepithelial resistance; Zonula-occludens.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures