ERO1A levels are a prognostic indicator in EGFR mutated non small cell lung cancer
- PMID: 39496753
- PMCID: PMC11535241
- DOI: 10.1038/s41698-024-00736-1
ERO1A levels are a prognostic indicator in EGFR mutated non small cell lung cancer
Abstract
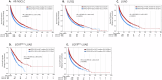
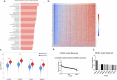
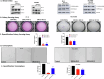
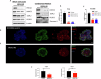
We have identified endoplasmic reticulum oxidoreductase 1 alpha (ERO1A) as a poor prognostic indicator in epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (EGFRMUT-NSCLC). In addition, comparison of high versus low ERO1A expression among cohorts of EGFRMUT-NSCLC primary samples revealed that ERO1A expression correlated with increased expression of proteins that regulate secretion. Using the CPTAC proteomic data set in lung adenocarcinoma we found that high ERO1A protein expression correlated with both extracellular matrix and matrix modifying enzymes. In this report, we found that ablating ERO1A expression was a determinant of clonogenicity, tumor sphere formation, spheroid growth and growth in vivo, as well as response to Osimertinib. We validated that ERO1A-knockout EGFRMUT-LUAD cell lines demonstrated a reduction in secretion of both laminin gamma 2 (LAMC2) and the collagen modifying enzyme lysyl oxidase-like 2 (LOXL2). Our work supports the role of ERO1A in modulating the tumor microenvironment that is likely to contribute to tumor progression.
© 2024. The Author(s).
Conflict of interest statement
L.A.H. is co-founder of Modulation Therapeutics but this work is not related to the current pipeline.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

