The role of cydB gene in the biofilm formation by Campylobacter jejuni
- PMID: 39496766
- PMCID: PMC11535028
- DOI: 10.1038/s41598-024-77556-7
The role of cydB gene in the biofilm formation by Campylobacter jejuni
Abstract
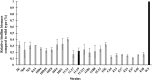
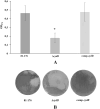
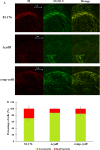
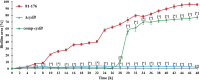
Campylobacter jejuni is a major cause of food- and water-borne bacterial infections in humans. A key factor helping bacteria to survive adverse environmental conditions is biofilm formation ability. Nonetheless, the molecular basis underlying biofilm formation by C. jejuni remains poorly understood. Around thirty genes involved in the regulation and dynamics of C. jejuni biofilm formation have been described so far. We applied random transposon mutagenesis to identify new biofilm-associated genes in C. jejuni strain 81-176. Of 1350 mutants, twenty-four had a decreased ability to produce biofilm compared to the wild-type strain. Some mutants contained insertions in genes previously reported to affect the biofilm formation process. The majority of identified genes encoded hypothetical proteins. In the library of EZ-Tn5 insertion mutants, we found the cydB gene associated with respiration that was not previously linked with biofilm formation in Campylobacter. To study the involvement of the cydB gene in biofilm formation, we constructed a non-marked deletion cydB mutant together with a complemented mutant. We found that the cydB deletion-mutant formed a weaker biofilm of loosely organized structure and lower volume than the parent strain. In the present study, we demonstrated the role of the cydB gene in biofilm formation by C. jejuni.
Keywords: C. jejuni; cydB; Biofilm; Transposon mutagenesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interests.
Figures


Update of
-
The role of cydB gene in the biofilm formation by Campylobacter jejuni.Res Sq [Preprint]. 2024 Sep 10:rs.3.rs-4342718. doi: 10.21203/rs.3.rs-4342718/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Nov 4;14(1):26574. doi: 10.1038/s41598-024-77556-7. PMID: 39315276 Free PMC article. Updated. Preprint.
References
-
- Jamal, M., Tasneem, U., Hussain, T. & Andleeb, S. Bacterial biofilm: its composition, formation and role in human infections. Res Rev J Microbiol Biotechnol4, (2015).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

