The Value of Primary Tumor Resection in Patients with Liver Metastases: A 10-Year Outcome
- PMID: 39496900
- PMCID: PMC11698763
- DOI: 10.1245/s10434-024-16386-3
The Value of Primary Tumor Resection in Patients with Liver Metastases: A 10-Year Outcome
Abstract
Objective: This study aimed to analyze the impact of primary tumor resection (PTR) on the prognosis of four common primary tumors with liver metastases, and to develop a prognostic model to visualize the PTR benefit rate of patients with liver metastases.
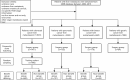
Materials and methods: Patients diagnosed with colorectal cancer liver metastases (CRLM), pancreatic cancer liver metastases (PLM), gastric cancer liver metastases (GLM), and breast cancer liver metastases (BLM) between 2004 and 2015 were retrospectively reviewed from the Surveillance, Epidemiology, and End Results (SEER) database and assigned to either the surgery or non-surgery groups. A 1:1 propensity score matching (PSM) was performed. Surgical patients who survived longer than the median cancer-specific survival (CSS) time for non-surgery patients constituted the benefit group. Logistic regression was conducted to explore the independent factors affecting surgical benefit, and a nomogram was established.
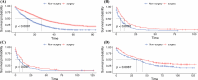
Results: A total of 21,928 patients with liver metastases were included. After PSM for surgery and non-surgery patients, we found that PTR had a significant impact on the overall survival (OS) and CSS of CRLM, PLM, and BLM patients. In CRLM patients, age (p < 0.001), primary site (p = 0.006), grade (p = 0.009), N stage (p = 0.034), and histology (p = 0.006) affected the surgical benefit. In BLM patients, the independent factors were age (p = 0.002), race (p = 0.020), and radiotherapy (p = 0.043). And in PLM patients, chemotherapy was an independent factor associated with a survival benefit from PTR.
Conclusion: PTR improved OS and CSS in patients with CRLM, PLM, and BLM. A predictive model was established to identify suitable candidates for PTR in CRLM patients.
Keywords: Liver metastases; Nomogram; Primary tumor resection; SEER.
© 2024. The Author(s).
Conflict of interest statement
Ethics Approval and Consent to Participate: All participants from our center signed written informed consent. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee at Shanghai East Hospital. Disclosure: Lin-Lin Liu, Yu-Kun Lin, and Zuo-Lin Xiang declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Benson AB, Venook AP, Al-Hawary MM, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(3):329-359. - PubMed
-
- Voss N, Izbicki JR, Nentwich MF. Oligometastases in pancreatic cancer (Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis). Ann Gastroenterol Surg. 2019;3(4):373–7. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- Grant No.PWZzk2022-02/Key Specialty Construction Project of Shanghai Pudong New Area Health Commission
- No.82160591/National Natural Science Foundation of China
- Grant No.23Y11909000/Shanghai Science and Technology Innovation Action Plan
- Grant No. PWR12023-02/Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai
- Grant No. DFLC2022012/Key Project of Clinical Research of Shanghai East Hospital, Tongji University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

