Site-specific drug release of monomethyl fumarate to treat oxidative stress disorders
- PMID: 39496929
- PMCID: PMC12049564
- DOI: 10.1038/s41587-024-02460-4
Site-specific drug release of monomethyl fumarate to treat oxidative stress disorders
Abstract
Treatment of diseases of oxidative stress through activation of the antioxidant nuclear factor E2-related factor 2 (NRF2) is limited by systemic side effects. We chemically functionalize the NRF2 activator monomethyl fumarate to require Baeyer-Villiger oxidation for release of the active drug at sites of oxidative stress. This prodrug reverses chronic pain in mice with reduced side effects and could be applied to other disorders of oxidative stress.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: T.D.A., J.L., D.J.L.T., A.D.A. and P.M.G. are named inventors on patent application WO/2021/179049 and provisional patent application 63/641,731 covering peroxide-activated prodrugs to treat disorders of oxidative stress. T.D.A, A.D.A. and P.M.G. received funding from Biogen. T.D.A., D.J.L.T., A.D.A. and P.M.G. founded and/or hold equity in ImmunoLogic, a start-up company that is developing pathology-activated prodrugs to treat diseases of oxidative stress. The other authors declare no competing interests.
Figures
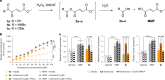


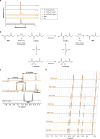
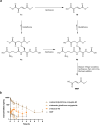
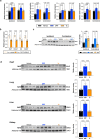

References
MeSH terms
Substances
Grants and funding
- UG3NS127251/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- UG3 NS127251/NS/NINDS NIH HHS/United States
- DP180101581/Department of Education and Training | Australian Research Council (ARC)
- CE140100003/Department of Education and Training | Australian Research Council (ARC)
- G12 MD007605/MD/NIMHD NIH HHS/United States
- RF1 NS113840/NS/NINDS NIH HHS/United States
- RF1NS113840/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P30 CA016672/CA/NCI NIH HHS/United States
- U.S. Department of Health & Human Services | National Institutes of Health/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RP180748/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
LinkOut - more resources
Full Text Sources
Medical

