Outcomes in hematopoetic cell transplantation in the setting of mold infections in patients with chronic granulomatous disease
- PMID: 39496936
- PMCID: PMC11810765
- DOI: 10.1038/s41409-024-02389-x
Outcomes in hematopoetic cell transplantation in the setting of mold infections in patients with chronic granulomatous disease
Abstract
Chronic granulomatous disease (CGD) is a disorder of immunity characterized by phagocyte dysfunction. Mold infections in patients with CGD are often severe and disseminated. We present patient characteristics, microbiological data, and outcomes for 26 patients with CGD who received hematopoietic cell transplantation (HCT) or gene therapy-modified cells (GT) between 2008 and 2019, with proven fungal infection either before or during their transplant. All patients engrafted, and all but one GT recipient had neutrophil recovery and evidence of functional correction. Eighteen patients (69%) are currently alive and 19 patients (73% of total, 90% of patients with repeat imaging performed) had evidence of radiographic improvement. With 3 exceptions, deaths were not principally related to the fungal infection and duration of antecedent infection did not correlate with death. Aspergillus species accounted for the majority of disease (50%), followed by Phellinus species (18%). Osteomyelitis and disseminated disease were common, as only 11 patients (42%) had disease restricted to pneumonia. Triazole therapy was used in all 26 patients, with combination therapy used in 25 (96%). HCT or gene therapy, with appropriate antifungal therapy, are viable therapies for refractory fungal infections in patients with CGD.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All participants signed written informed consent forms including consent to publish images. The patents were participants separate clinical trials, all of which were separately approved by the NIH Institutional Review Board (Approval numbers 16-I-0032, 000186-I, 19-I-0080). All procedures were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Figures
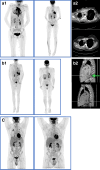

References
-
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. - DOI - PMC - PubMed
-
- Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–76. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

