Controlled release of hydrogel-encapsulated mesenchymal stem cells-conditioned medium promotes functional liver regeneration after hepatectomy in metabolic dysfunction-associated steatotic liver disease
- PMID: 39497124
- PMCID: PMC11536549
- DOI: 10.1186/s13287-024-03993-w
Controlled release of hydrogel-encapsulated mesenchymal stem cells-conditioned medium promotes functional liver regeneration after hepatectomy in metabolic dysfunction-associated steatotic liver disease
Abstract
Background: Globally, prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) is increasing, and there is an urgent need to develop innovative therapies that promote liver regeneration following hepatectomy for this disease. Surgical excision is a key therapeutic approach with curative potential for liver tumors. However, hepatic steatosis can lead to delayed liver regeneration and higher post-operative complication risk. Mesenchymal stem cells-conditioned medium (MSC-CM) is considered a rich source of paracrine factors that can repair tissues and restore function of damaged organs. Meanwhile, hydrogels have been widely recognized to load MSC secretome and achieve sustained release. This study aimed to evaluate the therapeutic effect of hydrogel-encapsulated MSC-CM on liver regeneration following partial hepatectomy (PHx) in a rodent model of diet-induced hepatic steatosis.
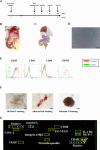
Methods: Male Lewis rats were fed with a methionine and choline-deficient diet. After 3 weeks of feeding, PHx was performed and rats were randomly allocated into two groups that received hydrogel-encapsulated MSC-CM or vehicle via the intra-mesenteric space of the superior mesenteric vein (SMV).
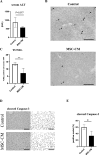
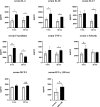
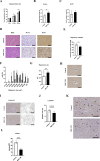
Results: The regeneration of the remnant liver at 30 and 168 h after PHx was significantly accelerated, and the expressions of proliferating cell nuclear antigen were significantly enhanced in the MSC-CM group. MSC-CM treatment significantly increased hepatic ATP and β-hydroxybutyrate content at 168 h after PHx, indicating that MSC-CM fosters regeneration not only in volume but also in functionality. The number of each TUNEL- and cleaved caspase-3 positive nuclei in hepatocytes at 9 h after PHx were significantly decreased in the MSC-CM group, suggesting that MSC-CM suppressed apoptosis. MSC-CM increased serum immunoregulatory cytokine interleukin-10 and interleukin-13 at 30 h after PHx. Additionally, mitotic figures and cyclin D1 expression decreased and hepatocyte size increased in the MSC-CM group, implying that this mode of regeneration was mainly through cell hypertrophy rather than cell division.
Conclusions: MSC-CM represents a novel therapeutic approach for patients with MASLD requiring PHx.
Keywords: Adipose tissue-derived mesenchymal stem cells; Conditioned medium; Controlled release; Hepatectomy; Hydrogel; Liver regeneration; Non-alcoholic fatty liver disease; Regenerative medicine.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The author(s) declare no competing interests.
Figures

References
-
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. - PubMed
-
- Henry L, Paik J, Younossi ZM. Review article: the epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. 2022;56:942–56. - PubMed
-
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–64. - PubMed
-
- Colle I, Verhelst X, Vanlander A, Geerts A, Van Vlierberghe H, Berrevoet F, Rogiers X, Troisi RI. Pathophysiology and management of post resection liver failure. Acta Chir Belg. 2013;113:155–61. - PubMed
-
- Kele PG, van der Jagt EJ, Gouw AS, Lisman T, Porte RJ, de Boer MT. The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver Int. 2013;33:469–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

