A nanoencapsulated oral formulation of fenretinide promotes local and metastatic breast cancer dormancy in HER2/neu transgenic mice
- PMID: 39497135
- PMCID: PMC11536529
- DOI: 10.1186/s13046-024-03213-6
A nanoencapsulated oral formulation of fenretinide promotes local and metastatic breast cancer dormancy in HER2/neu transgenic mice
Abstract
Background: Prevention and treatment of metastatic breast cancer (BC) is an unmet clinical need. The retinoic acid derivative fenretinide (FeR) was previously evaluated in Phase I-III clinical trials but, despite its excellent tolerability and antitumor activity in preclinical models, showed limited therapeutic efficacy due to poor bioavailability. We recently generated a new micellar formulation of FeR, Bionanofenretinide (Bio-nFeR) showing enhanced bioavailability, low toxicity, and strong antitumor efficacy on human lung cancer, colorectal cancer, and melanoma xenografts. In the present study, we tested the effect of Bio-nFeR on a preclinical model of metastatic BC.
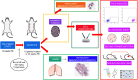
Methods: We used BC cell lines for in vitro analyses of cell viability, cell cycle and migratory capacity. For in vivo studies, we used HER2/neu transgenic mice (neuT) as a model of spontaneously metastatic BC. Mice were treated orally with Bio-nFeR and at sacrifice primary and metastatic breast tumors were analyzed by histology and immunohistochemistry. Molecular pathways activated in primary tumors were analyzed by immunoblotting. Stem cell content was assessed by flow cytometry, immunoblotting and functional assays such as colony formation ex vivo and second transplantation assay in immunocompromised mice.
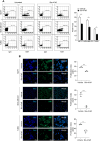
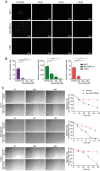
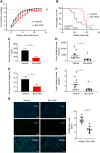
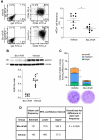
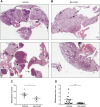
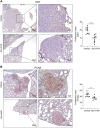
Results: Bio-nFeR inhibited the proliferation and migration of neuT BC cells in vitro and showed significant efficacy against BC onset in neuT mice. Importantly, Bio-nFeR showed the highest effectiveness against metastatic progression, counteracting both metastasis initiation and expansion. The main mechanism of Bio-nFeR action consists of promoting tumor dormancy through a combined induction of antiproliferative signals and inhibition of the mTOR pathway.
Conclusion: The high effectiveness of Bio-nFeR in the neuT model of mammary carcinogenesis, coupled with its low toxicity, indicates this formulation as a potential candidate for the treatment of metastatic BC and for the adjuvant therapy of BC patients at high risk of developing metastasis.
Keywords: Breast cancer; Cancer stem cells; Fenretinide; Metastasis; Metastatic dormancy; Retinoids; Tumor dormancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Harbeck N, Gnant M. Breast cancer. Lancet (London England). 2017;389(10074):1134–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

