COVID-19 vaccination modified the effect of nirmatrelvir-ritonavir on post-acute mortality and rehospitalization: a retrospective cohort study
- PMID: 39497519
- PMCID: PMC11539398
- DOI: 10.1080/22221751.2024.2421397
COVID-19 vaccination modified the effect of nirmatrelvir-ritonavir on post-acute mortality and rehospitalization: a retrospective cohort study
Abstract
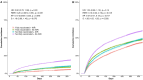
While previous research examined coronavirus disease 2019 (COVID-19) antiviral-vaccine interactions through exploratory subgroup analysis, none specifically designed for examining this interaction or its impact on post-acute outcomes. This study examined the interaction between nirmatrelvir-ritonavir and complete COVID-19 vaccination on reducing the risk of post-acute outcomes among COVID-19 patients. We followed COVID-19 patients hospitalized between 11 March 2022 and 10 October 2023, until 31 October 2023 in Hong Kong. Exposure groups were based on nirmatrelvir-ritonavir usage and vaccination status (fully or not fully vaccinated). Post-acute death and all-cause rehospitalization were the study outcomes. Propensity score weighting was applied to balance covariates among exposure groups, including age, sex, Charlson Comorbidity Index, and concomitant treatments. Multiplicative and additive interactions between nirmatrelvir-ritonavir and vaccination status were assessed. A total of 50,438 COVID-19 patients were included in this study and arranged into four exposure groups. Significant additive interaction on post-acute rehospitalization was observed (relative excess risk, 0.10; 95% CI, 0.02-0.19; p-value, 0.018; attributable proportion, 0.07; 95% CI, 0.01-0.12; p-value, 0.017; synergy index, 1.26; 95% CI, 1.02-1.55; p-value, 0.032). The interaction on post-acute mortality was marginally significant. In the subgroup analysis, the interaction effect is more pronounced in older adults, female, and CoronaVac recipients. In conclusion, our study demonstrated an additive interaction between nirmatrelvir-ritonavir and complete vaccination on post-acute outcomes, suggesting greater long-term benefits of the antiviral for fully vaccinated individuals compared to not fully vaccinated patients.
Keywords: Paxlovid; antiviral; interaction; long covid; post-covid.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- US FDA . Fact sheet for healthcare providers: emergency use authorization for paxlovid. [cited 2023 Dec 28] Available from: https://www.fda.gov/media/155050/download.
-
- Wong CKH, Au ICH, Lau KTK, et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–1693. doi: 10.1016/S1473-3099(22)00507-2 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
