Revolutionizing medicine: recent developments and future prospects in stem-cell therapy
- PMID: 39497543
- PMCID: PMC11634165
- DOI: 10.1097/JS9.0000000000002109
Revolutionizing medicine: recent developments and future prospects in stem-cell therapy
Abstract
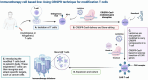
Stem-cell therapy is a revolutionary frontier in modern medicine, offering enormous capacity to transform the treatment landscape of numerous debilitating illnesses and injuries. This review examines the revolutionary frontier of treatments utilizing stem cells, highlighting the distinctive abilities of stem cells to undergo regeneration and specialized cell differentiation into a wide variety of phenotypes. This paper aims to guide researchers, physicians, and stakeholders through the intricate terrain of stem-cell therapy, examining the processes, applications, and challenges inherent in utilizing stem cells across diverse medical disciplines. The historical journey from foundational contributions in the late 19th and early 20th centuries to recent breakthroughs, including ESC isolation and iPSC discovery, has set the stage for monumental leaps in medical science. Stem cells' regenerative potential spans embryonic, adult, induced pluripotent, and perinatal stages, offering unprecedented therapeutic opportunities in cancer, neurodegenerative disorders, cardiovascular ailments, spinal cord injuries, diabetes, and tissue damage. However, difficulties, such as immunological rejection, tumorigenesis, and precise manipulation of stem-cell behavior, necessitate comprehensive exploration and innovative solutions. This manuscript summarizes recent biotechnological advancements, critical trial evaluations, and emerging technologies, providing a nuanced understanding of the triumphs, difficulties, and future trajectories in stem cell-based regenerative medicine. Future directions, including precision medicine integration, immune modulation strategies, advancements in gene-editing technologies, and bioengineering synergy, offer a roadmap in stem cell treatment. The focus on stem-cell therapy's potential highlights its significant influence on contemporary medicine and points to a future in which individualized regenerative therapies will alleviate various medical disorders.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
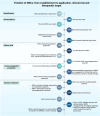
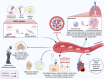
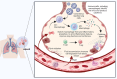
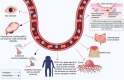
Figures

References
-
- Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 2015;17:11–22. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. - PubMed
-
- Hansford S, Huntsman DG. Boveri at 100: Theodor Boveri and genetic predisposition to cancer. J Pathol 2014;234:142–145. - PubMed
-
- Maximow AA. Der lymphozyt als gemeinsame stammzelle der verschiedenen blutelemente in der embryonalen entwicklung und im postfetalen leben der säugetiere. Cellul Ther Transplantat 2009;1:9–13.
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154–156. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

