Shock wave-pretreated ADMSCs of cell-sheet scaffold (CSS) patched on the left ventricular wall (LVW) inhibited LVW remodeling in mini-pig MI: role of CSS on counteracting Laplace's Law of LVW stress - experimental study
- PMID: 39497545
- PMCID: PMC11634124
- DOI: 10.1097/JS9.0000000000002119
Shock wave-pretreated ADMSCs of cell-sheet scaffold (CSS) patched on the left ventricular wall (LVW) inhibited LVW remodeling in mini-pig MI: role of CSS on counteracting Laplace's Law of LVW stress - experimental study
Abstract
Background: We investigated whether shock wave (SW)-pretreated autologous adipocyte-derived mesenchymal stem cells (ADMSCs) seeded in the cell-sheet scaffold (CSS) could inhibit left ventricular (LV) remodeling and improve LV ejection fraction (LVEF) in old myocardial infarction (MI).
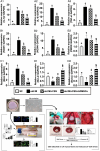
Methods: Mini-pigs ( n =20) were divided into group 1 (sham-operated control), group 2 (old MI), group 3 (old MI + autologous ADMSCs/1.0×10 7 in CSS on LV myocardium), and group 4 [old MI + SW (0.12 mJ/mm 2 for total 140 shots)-pretreated ADMSCs in CSS on LV myocardium]. Treatments started on day 28 after MI induction. In-vivo and in-vitro studies were conducted.
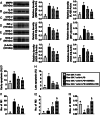
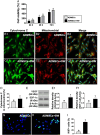
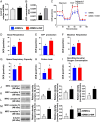
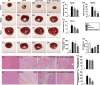
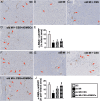
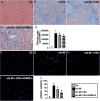
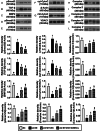
Results: Cell viability/relative mitochondria DNA expression/mitochondrial cytochrome C/adenosine triphosphate concentration in ADMCSs and protein expressions of angiogenesis factors [vascular endothelial growth factor (VEGF)/stromal cell-derived factor-1 (SDF-1)/mitochondrial respiratory chain complexes I-IV/oxygen consumption rate] were higher in group 4 than in group 3 ( P <0.001). By day 180, LVEF and small vessel numbers in the peri-infarct or infarct area were highest in group 1, lowest in group 2, and significantly lower in group 3 than in group 4. In contrast, the LV dimension was opposite to the pattern of change in LVEF in all groups ( P <0.0001). The basal/middle/apical infarct and fibrotic areas were inversely related to LVEF in all groups (all P <0.0001). Protein levels of angiogenetic markers (SDF-1α/C-X-C chemokine receptor type 4/VEGF/angiopoietin-1) were significantly and persistently increased from groups 1 to 4. In contrast, protein levels of endothelial cell markers (von Willebrand factor or endothelial nitric oxide synthase) showed an identical pattern to LVEF in all groups (all P <0.0001).
Conclusion: SW pretreatment of ADMSCs seeded in CSS offered significant benefits in preserving LV performance and ameliorating LV remodeling in mini-pigs with old MI.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Nordlander R, Nyquist O. Mortality, arrhythmias and pump failure in acute myocardial infarction in relation to estimated infarct size. Acta Med Scand 1979;206:65–71. - PubMed
-
- Erlebacher JA, Weiss JL, Weisfeldt ML, et al. Early dilation of the infarcted segment in acute transmural myocardial infarction: role of infarct expansion in acute left ventricular enlargement. J Am Coll Cardiol 1984;4:201–208. - PubMed
-
- Pfeffer MA, Lamas GA, Vaughan DE, et al. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med 1988;319:80–86. - PubMed
-
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669–677. - PubMed
-
- Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation 1999;100:999–1008. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

