Nationwide Target Trial Emulation Evaluating the Clinical Effectiveness of Oral Antivirals for COVID-19 in Korea
- PMID: 39497563
- PMCID: PMC11538573
- DOI: 10.3346/jkms.2024.39.e272
Nationwide Target Trial Emulation Evaluating the Clinical Effectiveness of Oral Antivirals for COVID-19 in Korea
Abstract
Background: Despite the proven effectiveness of oral antivirals against severe acute respiratory syndrome coronavirus 2 in randomized trials, their clinical reevaluation is vital in the context of widespread immunity and milder prevalent variants. This study aimed to assess the effectiveness of oral antivirals for coronavirus disease 2019 (COVID-19).
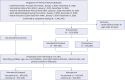
Methods: This retrospective cohort study utilized a target trial emulation framework to analyze patients with COVID-19 aged 60+ from January to December 2022. Data were obtained from the Korea Disease Control and Prevention Agency and Health Insurance Review and Assessment Service. The study involved 957,036 patients treated with nirmatrelvir/ritonavir and 243,360 treated with molnupiravir, each compared with the matched control groups. Primary outcome was progression to critical COVID-19 requiring advanced respiratory support. Secondary outcomes included progression to severe COVID-19, need for supplemental oxygen, and death within 30 days of the onset of COVID-19. Number needed to treat (NNT) derived from the absolute risk reduction.
Results: Nirmatrelvir/ritonavir was significantly associated with a reduced risk of severe (adjusted odds ratio [aOR], 0.823; 95% confidence interval [CI], 0.803-0.843), critical (aOR, 0.560; 95% CI, 0.503-0.624), and fatal COVID-19 (aOR, 0.694; 95% CI, 0.647-0.744). Similarly, molnupiravir reduced the risk of severe (aOR, 0.895; 95% CI, 0.856-0.937), critical (aOR, 0.672; 95% CI, 0.559-0.807), and fatal cases (aOR, 0.679; 95% CI, 0.592-0.779). NNTs for nirmatrelvir/ritonavir were 203.71 (severe), 1,230.12 (critical), and 691.50 (death); for molnupiravir, they were 352.70 (severe), 1,398.62 (critical), and 862.98 (death). Higher effectiveness was associated with older adults, unvaccinated individuals, and the late pandemic phase.
Conclusion: Nirmatrelvir/ritonavir and molnupiravir are effective in preventing progression to severe disease in elderly adults with COVID-19.
Keywords: COVID-19; Effectiveness; Oral Antivirals; SARS-CoV-2; Target Trial Emulation.
© 2024 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Tan TT, Ng HJ, Young B, Khan BA, Shetty V, Azmi N, et al. Effectiveness of vaccination against SARS-CoV-2 and the need for alternative preventative approaches in immunocompromised individuals: a narrative review of systematic reviews. Expert Rev Vaccines. 2023;22(1):341–365. - PubMed
-
- Pfizer. Highlights of prescribing information (paxlovid) [Updated 2023]. [Accessed December 10, 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217188s000lbl.pdf .
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous