Effects of Cimetidine and Dolutegravir on the Endogenous Drug-Drug Interaction Biomarkers for Organic Cation Transporter 2 and Multidrug and Toxin Extrusion Protein 1 in Healthy Volunteers
- PMID: 39497599
- PMCID: PMC11739737
- DOI: 10.1002/cpt.3482
Effects of Cimetidine and Dolutegravir on the Endogenous Drug-Drug Interaction Biomarkers for Organic Cation Transporter 2 and Multidrug and Toxin Extrusion Protein 1 in Healthy Volunteers
Abstract
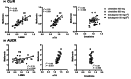
This study was designed to assess the quantitative performance of endogenous drug-drug interaction (DDI) biomarkers (N1-methylnicotinamide (1-NMN), N1-methyladenosine (m1A), and creatinine) for the organic cation transporters, OCT2 and MATE1/2K in the kidney. Ten healthy volunteers received cimetidine (400 and 800 mg, single dose) or dolutegravir (50 mg, twice a day) together with metformin (500 mg). Cimetidine and dolutegravir were considered to act mainly as MATE1/2K and OCT2 inhibitors, respectively. The renal clearance (CLr) of metformin was decreased by 15.5% and 42.5% by cimetidine 400 and 800 mg, and by 26.8% and 56.9% by dolutegravir first and fifth doses, respectively. CLr ratio (CLrR) of 1-NMN were 0.93 and 0.64 for cimetidine 400 and 800 mg, and 0.87 and 0.47 for dolutegravir first and fifth doses, respectively. CLrR of m1A was less than that of 1-NMN: 1.0 and 0.80 for cimetidine 400 and 800 mg, and 0.77 and 0.71 for dolutegravir first and fifth doses, respectively. CLr of creatinine was significantly decreased only by cimetidine 800 mg. Individual CLrR of 1-NMN and m1A showed a positive correlation with the corresponding CLrR of metformin with r2 of 0.58 and 0.55, respectively. When evaluated individually, m1A showed a better correlation during cimetidine periods (r2 0.64) than 1-NMN (r2 0.36), but vice versa during dolutegravir periods (r2 1-NMN, 0.80; m1A, 0.32). These results suggest that 1-NMN and m1A might be more promising than creatinine as endogenous biomarkers for quantitatively assessing the DDI potential of investigational drugs for OCT2 and MATE1/2K based on their CLrR change.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Hideki Hirabayashi is employed by Takeda pharmaceutical company and hold common stocks of Takeda pharmaceutical company. All other authors declared no competing interests for this work.
Figures

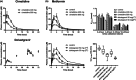


References
-
- Kikuchi, R. et al. Utilization of OATP1B biomarker coproporphyrin‐I to guide drug‐drug interaction risk assessment: evaluation by the pharmaceutical industry. Clin. Pharmacol. Ther. 114, 1170–1183 (2023). - PubMed
-
- Mochizuki, T. & Kusuhara, H. Progress in the quantitative assessment of transporter‐mediated drug–drug interactions using endogenous substrates in clinical studies. Drug Metab. Dispos. 51, 1105–1113 (2023). - PubMed
-
- Muller, F. , Sharma, A. , Konig, J. & Fromm, M.F. Biomarkers for in vivo assessment of transporter function. Pharmacol. Rev. 70, 246–277 (2018). - PubMed
-
- Zamek‐Gliszczynski, M.J. et al. ITC commentary on metformin clinical drug–drug interaction study design that enables an efficacy‐ and safety‐based dose adjustment decision. Clin. Pharmacol. Ther. 104, 781–784 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

