Pharmacokinetic/pharmacodynamic analysis of geographic atrophy lesion area in patients receiving pegcetacoplan treatment or sham
- PMID: 39497616
- PMCID: PMC11812941
- DOI: 10.1002/psp4.13264
Pharmacokinetic/pharmacodynamic analysis of geographic atrophy lesion area in patients receiving pegcetacoplan treatment or sham
Abstract
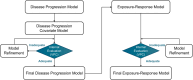
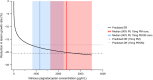
Pegcetacoplan is a complement C3/C3b inhibitor indicated for the treatment of geographic atrophy (GA). A population pharmacokinetic (PK)/pharmacodynamic (PD) analysis of pegcetacoplan used GA lesion area measurements from three clinical studies to determine the effect of pegcetacoplan exposure on GA progression. A base disease progression model was developed using data from sham-treated eyes and untreated fellow eyes, followed by treatment effect assessment in dose-response and PK/PD models. In total, 1501 patients from FILLY (NCT02503332), OAKS (NCT03525613), and DERBY (NCT03525600) received intravitreal pegcetacoplan 15 mg monthly or every other month (EOM) or sham treatment monthly or EOM and were included in the population analysis of lesion area. Disease progression over time was adequately described as linear-with-time over the 24-month maximal study duration. Disease-specific covariates associated with slower lesion growth were unilateral, unifocal, and subfoveal GA lesions and >20 intermediate or large drusen groups (≥63 μm) at baseline. The dose-response model estimated 0.80-fold (95% CI: 0.75, 0.84) and 0.83-fold (95% CI: 0.78, 0.87) reductions in GA lesion growth rate with pegcetacoplan monthly and EOM, respectively, versus sham. A relationship between vitreous humor concentration and GA lesion growth rate was quantified as 2.6% per unit of log-transformed vitreous pegcetacoplan concentration in the PK/PD model. PK/PD predictions of treatment effect based on exposure (pegcetacoplan monthly: 0.80 [90% CI: 0.77, 0.84]; pegcetacoplan EOM: 0.83 [90% CI: 0.80, 0.86]) were consistent with predictions based on dose response. These results support the benefit of pegcetacoplan administered monthly or EOM in slowing GA lesion growth.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CRB is an employee of Apellis Pharmaceuticals and may hold stock and/or stock options. RLC, KP, FG, and RR are former employees of Apellis and may hold stock and/or stock options. RLC, EL, and SC are paid consultants for A2‐Ai LLC, a full‐service clinical pharmacology consultancy service provider.
Figures




References
-
- Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age‐related macular degeneration. Ophthalmology. 2018;125(3):369‐390. - PubMed
-
- Singh RP, Patel SS, Nielsen JS, Schmier JK, Rajput Y. Patient‐, caregiver‐, and eye care professional‐reported burden of geographic atrophy secondary to age‐related macular degeneration. Am J Ophthmol Clin Trials. 2019;2(1):1.
-
- Holz FG, Strauss EC, Schmitz‐Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079‐1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

