Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease: final results from the phase II SOLACE-adults study
- PMID: 39497751
- PMCID: PMC11533247
- DOI: 10.1177/20406207241292508
Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease: final results from the phase II SOLACE-adults study
Abstract
Background: Crizanlizumab is a novel inhibitor of P-selectin, a key player in multicellular adhesion and inflammatory signaling, that leads to vaso-occlusion in sickle cell disease (SCD).
Objectives: The SOLACE-adults study evaluated the pharmacokinetics, pharmacodynamics (P-selectin inhibition), safety, and efficacy of crizanlizumab, with or without hydroxyurea/hydroxycarbamide, in patients with SCD.
Design: Phase II, single-arm, multicenter study.
Methods: Patients with SCD aged 16-70 years, with ⩾1 vaso-occlusive crisis (VOC) within 12 months before screening, received crizanlizumab 5.0 or 7.5 mg/kg intravenous infusion every 4 weeks; dose groups were enrolled sequentially.
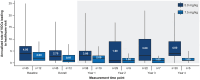
Results: Of 57 patients enrolled, 45 received crizanlizumab 5.0 mg/kg and 12 received 7.5 mg/kg for a median duration of 206 and 170 weeks, respectively. Crizanlizumab concentrations reached maximum levels after a 30-min infusion and remained steady for 6 h, without significant accumulation. P-selectin inhibition was nearly complete for both doses. The median (interquartile range) absolute change in the annualized rate of VOCs leading to healthcare visit from baseline was -0.79 (-3.04, 2.01) in the 5.0 mg/kg group and -0.98 (-1.11, -0.41) in the 7.5 mg/kg group. All patients experienced at least one adverse event (AE), with no apparent differences between the two doses in the frequency and severity of AEs. Grade ⩾3 AEs occurred in 60% of the 5.0 mg/kg group and 58% of the 7.5 mg/kg group. Two patients in the 5.0 mg/kg group and one in the 7.5 mg/kg group had severe crizanlizumab-related infusion-related reactions, which resolved with treatment. No patients developed antibodies against crizanlizumab.
Conclusion: Crizanlizumab 5.0 and 7.5 mg/kg demonstrated a dose-proportional increase in exposure, sustained P-selectin inhibition, a tolerable safety profile, and a sustained reduction in VOCs leading to healthcare visit. This suggests that crizanlizumab is a useful treatment option for patients with SCD who have experienced VOCs.
Trial registration: NCT03264989.
Keywords: crizanlizumab; pharmacodynamics; pharmacokinetics; sickle cell disease; vaso-occlusive crises.
Plain language summary
Understanding the effects of crizanlizumab in patients with sickle cell disease: Results from the SOLACE-adults study Crizanlizumab is a drug that inhibits P-selectin, which is involved in the inflammation and blockage of blood vessels that occurs in an inherited blood disorder called sickle cell disease (SCD). The SOLACE-adults study investigated the effects of crizanlizumab on the pharmacokinetics (how the drug moves through the body), pharmacodynamics (its effects on P-selectin), safety, and efficacy in patients with SCD. This was a Phase II study conducted at multiple centers, where all patients received crizanlizumab. Patients aged between 16 to 70 years who had SCD and experienced vaso-occlusive crises (VOCs, painful episodes caused by blocked blood vessels) within the year before the study were given crizanlizumab intravenously every 4 weeks at a dose of either 5.0 mg/kg or 7.5 mg/kg. A total of 57 patients enrolled in the study, of whom 45 received the 5.0 mg/kg dose and 12 received the 7.5 mg/kg dose. Crizanlizumab levels in the blood peaked 30 minutes after the infusion and remained steady for 6 hours. Both doses effectively inhibited P-selectin. The median reduction in the rate of vaso-occlusive crises leading to healthcare visits was -0.79 in the 5.0 mg/kg group and -0.98 in the 7.5 mg/kg group. All patients experienced adverse events, but there were no major differences between the two doses. Grade ⩾3 adverse events occurred in 60% of the 5.0 mg/kg group and 58% of the 7.5 mg/kg group. Some patients had severe reactions to the infusion, but these reactions resolved with treatment. None of the patients developed antibodies against crizanlizumab. In conclusion, crizanlizumab at both doses reached levels of exposure that caused sustained inhibition of P-selectin, had tolerable safety, and reduced VOCs requiring medical visits. This suggests that crizanlizumab is a beneficial treatment option for patients with SCD experiencing these painful crises.
© The Author(s), 2024.
Conflict of interest statement
J.K. reports receipt of research funding from NHLBI, BEAM, Bluebird Bio, CDC, HRSA, Novartis, Novo Nordisk, and Takeda; consultancy for Austin Pharmaceuticals, Bausch, BEAM, Bluebird Bio, ECOR-1, Fulcrum, Novartis, and Watkins, Lourie, Roll & Chance; membership on an entity’s Board of Directors or advisory committees with Austin Pharmaceuticals, Bluebird Bio, Ciesi, Glycomimetics, and Novartis; and honoraria from Guidepoint Global; D.M. reports consultancy from Editas, GBT, Novartis, Novo Nordisk, and Pfizer; A.K. reports receipt of research funding from Akira Bio, Forma/Novo-Nordisk, GBT/Pfizer, and Novartis; consultancy for Novartis; membership on an entity’s Board of Directors or advisory committees with Bluebird Bio, GBT/Pfizer, and Novartis; and event adjudication committee (EAC) Chair for Vertex; N.S. reports receipt of research funding from GBT/Pfizer; consultancy for Agios Pharmaceuticals, Bluebird Bio, Forma, GBT/Pfizer, and Vertex; and speaker bureau for Alexion Pharmaceuticals and GBT/Pfizer; D.L. reports clinical trial activity as principal investigator or sub-investigator for Annexon Biosciences Inc., Baxalta, BeiGene Ltd., Bioverativ Inc., Celgene, Delta, Exact Sciences, Janssen Research and Development, Immunovant Inc., Incyte Corp., Novartis, Partner Therapeutics, Principia Biopharma Inc., Sanofi-Aventis LLC, Takeda, and Vifor Pharma; D.K., M.N., and E.R. are employees of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA; H.M. is an employee of Novartis Healthcare Private Limited, Hyderabad, India; and A.E.M. is an employee of BioMedical Research, Cambridge, MA, USA; S.M. and S.M.N. have nothing to disclose.
Figures


References
-
- Karki NR, Saunders K, Kutlar AA. critical evaluation of crizanlizumab for the treatment of sickle cell disease. Expert Rev Hematol 2022; 15(1): 5–13. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

