Effective BPA degradation in water: the integration of bimetallic UiO-66 Ce-Zr
- PMID: 39497778
- PMCID: PMC11533980
- DOI: 10.1039/d4ra06460g
Effective BPA degradation in water: the integration of bimetallic UiO-66 Ce-Zr
Abstract
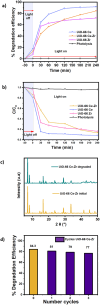
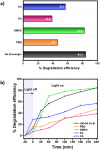
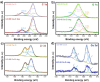
In this work, a bimetallic MOF UiO-66 Ce-Zr to degrade bisphenol A (BPA) in water was synthesised. The material exhibited a remarkable degradation efficiency of 84.3% under UV irradiation for 240 minutes. Combining cerium (Ce) and zirconium (Zr) in the MOF structure enhanced the catalytic activity and reinforced its structural stability. Comprehensive characterisation was performed using PXRD, FT-IR, SEM-EDS, XPS, and N₂ adsorption-desorption isotherms. Scavenger tests confirmed that hydroxyl (˙OH) and superoxide (˙O₂⁻) radicals played a crucial role in the photocatalysis. The material demonstrated excellent reusability, maintaining high performance over three cycles with minimal structural changes. Furthermore, a toxicological evaluation of the degradation by-products was conducted using UPLC-MS, reaffirming the potential of the material as an efficient water treatment system. This study underscores the potential of UiO-66 Ce-Zr as a stable and effective photocatalyst for water treatment applications, particularly in removing emerging pollutants such as BPA.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Currell M. J. Han D. Environment: Science and Policy for Sustainable Development. 2017;59:16–29.
-
- Geissen V. Mol H. Klumpp E. Umlauf G. Nadal M. Van Der Ploeg M. Van De Zee S. E. A. T. M. Ritsema C. J. Int. Soil Water Conserv. Res. 2015;3:57–65.
-
- Zhang S. Wang J. Zhang Y. Ma J. Huang L. Yu S. Chen L. Song G. Qiu M. Wang X. Environ. Pollut. 2021;291:118076. - PubMed
-
- Garcia-Segura S. Brillas E. J. Photochem. Photobiol., C. 2017;31:1–35.
LinkOut - more resources
Full Text Sources

