Rapid and selective quantitative colourimetric analysis of nitrite in water using a S-Nitrosothiol based method
- PMID: 39497788
- PMCID: PMC11533637
- DOI: 10.1016/j.wroa.2024.100265
Rapid and selective quantitative colourimetric analysis of nitrite in water using a S-Nitrosothiol based method
Abstract
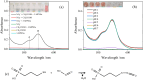
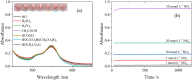
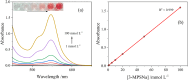
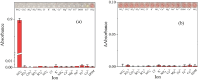
This study introduces a novel S-nitrosothiol based method for the rapid and highly selective detection of nitrite in complex water matrices. Sodium 3-mercapto-1-propanesulfonate forms a distinctive pink S-nitrosothiol compound upon interaction with nitrite in acidic media, allowing both visual and quantitative detection. Various factors affecting the absorbance of the final product were investigated, including pH, reaction time, acid type, and sodium 3-mercapto-1-propanesulfonate concentration. UV-Vis spectrophotometric analysis demonstrated an excellent linear correlation (R2 = 0.99) across a broad detection range (0.05 to 80 mmol l-1), while showing no interference from common ions such as nitrate or dissolved organic matter, a limitation frequently observed in conventional UV-based nitrite detection methods. The assay was further adapted into a pellet form to simplify field use, operating effectively at room temperature with a low detection limit (1.4 ppm). The S-nitrosothiol based method represents a safer and more environmentally friendly option for nitrite detection and shows a promising potential as a valuable addition to both field and laboratory water testing kits for nitrite analysis.
Keywords: Colourimetric detection; Nitrite; Wastewater; Water analysis.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Egle Latvyte, John Graves reports financial support was provided by 10.13039/501100000780European Commission. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bravo M., Olwiert A.C., Oelckers B. Nitrate determination in Chilean caliche samples by UV–Visible absorbance measurements and multivariate calibration. J. Chil. Chem. Soc. 2009;54:93–98. doi: 10.4067/S0717-97072009000100022. - DOI
-
- Currie L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities1. Anal. Chim. Acta. 1999;391:105–126. doi: 10.1016/S0003-2670(99)00104-X. - DOI
-
- Fan A.M. in: Encyclopedia of toxicology. Elsevier; 2014. Nitrate; pp. 523–527. - DOI
LinkOut - more resources
Full Text Sources

