Comparison of protective effects of teneligliptin and luseogliflozin on pancreatic β-cell function: randomized, parallel-group, multicenter, open-label study (SECRETE-I study)
- PMID: 39497800
- PMCID: PMC11532122
- DOI: 10.3389/fendo.2024.1412553
Comparison of protective effects of teneligliptin and luseogliflozin on pancreatic β-cell function: randomized, parallel-group, multicenter, open-label study (SECRETE-I study)
Abstract
Aims: The aim of this study is to directly compare the effects of SGLT2 inhibitors and DPP-4 inhibitors on β-cell function in patients with type 2 diabetes.
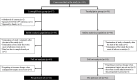
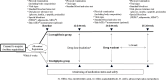
Materials and methods: We conducted a 26-week, randomized, open-label, parallel-group study, including a 1-2 week drug washout period, in patients with type 2 diabetes with HbA1c ≥7.0% and <9.0% and BMI ≥20 kg/m2 despite treatment with a drug naïve or other than DPP-4 inhibitors or SGLT2 inhibitors. A total of 103 subjects were randomly assigned to receive once daily oral luseogliflozin (L) or teneligliptin (T). The primary endpoint was the effect of L vs. T on the change in logarithmus naturalis (Ln) disposition index (DI) (DI 0-120min; combining measures of insulin secretion and sensitivity) from baseline to week 25-26 (post intervention), which was calculated by conducting an oral glucose tolerance test.
Results: Ln DI 0-120min were improved in both groups: -0.46 ± 0.68 to -0.20 ± 0.59 (p=0.03) in L group and -0.26 ± 0.60 to -0.05 ± 0.62 (p=0.01) in T group. The change in Ln serum proinsulin/C-peptide ratio, a marker of β-cell dysfunction, was reduced in L group (1.63 ± 0.63 to 1.56 ± 0.68, p=0.16), but rather increased in T group (1.70 ± 0.75 to 1.90 ± 0.51, p=0.01), with significant difference between the two groups (-0.27; p=0.004).
Conclusions: Improvement of disposition index in subjects with obese type 2 diabetes was comparable between luseogliflozin and teneligliptin. On the other hand, it is likely that alleviation of β-cell dysfunction is more effective with luseogliflozin compared to tenegliptin.
Clinical trial registration: https://rctportal.niph.go.jp/en, identifier jRCTs061190008.
Keywords: DPP-4 inhibitor; SGLT2 inhibitor; proinsulin; type 2 diabetes; β-cell function.
Copyright © 2024 Shimoda, Katakura, Mashiko, Iwamoto, Nakanishi, Anno, Kawasaki, Obata, Fushimi, Sanada, Kohara, Isobe, Iwamoto, Hirukawa, Tatsumi, Kimura, Kimura, Mune, Kaku and Kaneto.
Conflict of interest statement
HK has received honoraria for lectures and received scholarship grants from Sanofi, Novo Nordisk, Lilly, Boehringer Ingelheim, MSD, Takeda, Ono Pharma, Daiichi Sankyo, Sumitomo Pharma, Mitsubishi Tanabe Pharma, Pfizer, Kissei Pharma, AstraZeneca, Astellas, Novartis, Kowa, Chugai and Taisho Pharma. KKa has been an advisor to, received honoraria for lectures from, and received scholarship grants from Novo Nordisk Pharma, Sanwa Kagaku Kenkyusho, Takeda, Taisho Pharmaceutical Co., Ltd, MSD, Kowa, Sumitomo Pharma, Novartis, Mitsubishi Tanabe Pharma, AstraZeneca, Nippon Boehringer Ingelheim Co., Ltd, Chugai, Daiichi Sankyo, and Sanofi. TK has received honoraria for lectures from Sumitomo Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ministry of Health, Labour and Welfare of Japan . The national health and nutrition survey (2016). Available online at: http://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html (Accessed 31st October, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

