Analysis of lipid uptake, storage, and fatty acid oxidation by group 2 innate lymphoid cells
- PMID: 39497825
- PMCID: PMC11532145
- DOI: 10.3389/fimmu.2024.1493848
Analysis of lipid uptake, storage, and fatty acid oxidation by group 2 innate lymphoid cells
Abstract
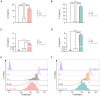
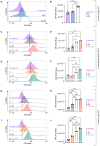
Group 2 Innate Lymphoid Cells (ILC2) are critical drivers of both innate and adaptive type 2 immune responses, known to orchestrate processes involved in tissue restoration and wound healing. In addition, ILC2 have been implicated in chronic inflammatory barrier disorders in type 2 immunopathologies such as allergic rhinitis and asthma. ILC2 in the context of allergen-driven airway inflammation have recently been shown to influence local and systemic metabolism, as well as being rich in lipid-storing organelles called lipid droplets. However, mechanisms of ILC2 lipid anabolism and catabolism remain largely unknown and the impact of these metabolic processes in regulating ILC2 phenotypes and effector functions has not been extensively characterized. ILC2 phenotypes and effector functions are shaped by their metabolic status, and determining the metabolic requirements of ILC2 is critical in understanding their role in type 2 immune responses and their associated pathophysiology. We detail here a novel experimental method of implementing flow cytometry for large scale analysis of fatty acid uptake, storage of neutral lipids, and fatty acid oxidation in primary murine ILC2 with complementary morphological analysis of lipid storage using confocal microscopy. By combining flow cytometry and confocal microscopy, we can identify the metabolic lipid requirements for ILC2 functions as well as characterize the phenotype of lipid storage in ILC2. Linking lipid metabolism pathways to ILC2 phenotypes and effector functions is critical for the assessment of novel pharmaceutical strategies to regulate ILC2 functions in type 2 immunopathologies.
Keywords: fatty acid oxidation (FAO); fatty acid uptake; flow cytometry; group 2 innate lymphoid cells (ILC2); immunometabolism; lipid droplets; microscopy; type 2 immunity.
Copyright © 2024 Roy-Dorval, Deagle, Roth, Raybaud, Ismailova, Krisna, Aboud, Stegen, Leconte, Berberi, Esomojumi and Fritz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. JHF declares that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

