Role of artificial intelligence in staging and assessing of treatment response in MASH patients
- PMID: 39497843
- PMCID: PMC11532183
- DOI: 10.3389/fmed.2024.1480866
Role of artificial intelligence in staging and assessing of treatment response in MASH patients
Abstract
Background and aims: The risk of disease progression in MASH increases proportionally to the pathological stage of fibrosis. This latter is evaluated through a semi-quantitative process, which has limited sensitivity in reflecting changes in disease or response to treatment. This study aims to test the clinical impact of Artificial Intelligence (AI) in characterizing liver fibrosis in MASH patients.
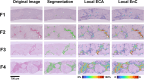
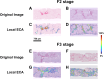
Methods: The study included 60 patients with clinical pathological diagnosis of MASH. Among these, 17 received a medical treatment and underwent a post-treatment biopsy. For each biopsy (n = 77) a Sirius Red digital slide (SR-WSI) was obtained. AI extracts >30 features from SR-WSI, including estimated collagen area (ECA) and entropy of collagen (EnC).
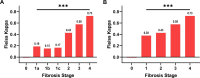
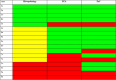
Results: AI highlighted that different histopathological stages are associated with progressive and significant increase of ECA (F2: 2.6% ± 0.4; F3: 5.7% ± 0.4; F4: 10.9% ± 0.8; p: 0.0001) and EnC (F2: 0.96 ± 0.05; F3: 1.24 ± 0.06; F4: 1.80 ± 0.11, p: 0.0001); disclosed the heterogeneity of fibrosis among pathological homogenous cases; revealed post treatment fibrosis modification in 76% of the cases (vs 56% detected by histopathology).
Conclusion: AI characterizes the fibrosis process by its true, continuous, and non-categorical nature, thus allowing for better identification of the response to anti-MASH treatment.
Keywords: MASH; artificial intelligence; fibrosis; liver; treatment.
Copyright © 2024 Akpinar, Panzeri, De Carlo, Belsito, Durante, Chirico, Lombardi, Fracanzani, Maggioni, Arcari, Roncalli, Terracciano, Inverso, Aghemo, Pugliese, Sironi and Di Tommaso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. (2015) 149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

