Delineating the nexus between gut-intratumoral microbiome and osteo-immune system in bone metastases
- PMID: 39497943
- PMCID: PMC11532283
- DOI: 10.1016/j.bonr.2024.101809
Delineating the nexus between gut-intratumoral microbiome and osteo-immune system in bone metastases
Abstract
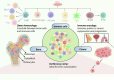
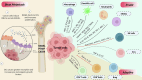
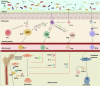
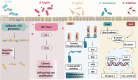
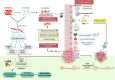
Emerging insights in osteoimmunology have enabled researchers to explore in depth the role of immune modulation in regulating bone health. Bone is one of the common sites of metastasis notably in case of breast cancer, prostate cancer and several other cancer types. High calcium ion concentration and presence of several factors within the mineralized bone matrix including TGF-β, BMP etc., aid in tumor growth and proliferation. Accumulating evidence has substantiated the role of the gut-microbiota (GM) in tumorigenesis, further providing a strong impetus for the growing "immune-cancer-gut microbiota" relationship. Recent advancements in research further highlight the importance of the intra-tumor microbiota in conjunction with GM in cancer metastasis. Intratumoral microbiota owing to their ability to cause genetic instability, mutations, and epigenetic modifications within the tumor microenvironment, has been recognized to affect cancer cell physiology. The host microbiota and immune system crosstalk shapes the innate and adaptive arms of the immune system, which is the key player in cancer progression. In this review, we aim to decipher the role of microorganisms mediating bone metastasis by shedding light on the immuno-onco-microbiome (IOM) axis. We discussed the feasible cancer therapeutic interventions based on the modulation of the microbiome-immune cell axis which includes prebiotics, probiotics, and postbiotics. Here, we leverage the conceptual framework based on the published articles on microbiota-based therapies to target bone metastases. Understanding this complicated nexus will provide insights into fundamental factors governing bone metastases which will subsequently help in managing this malignancy with better efficacy.
Keywords: Biotics; Bone cancer; Bone metastases; Gut microbiota; Immune system; Intratumoral microbiota; Osteo-immuno-oncology.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Amary M.F., Bacsi K., Maggiani F., Damato S., Halai D., Berisha F., Pollock R., O’Donnell P., Grigoriadis A., Diss T., Eskandarpour M., Presneau N., Hogendoorn P.C., Futreal A., Tirabosco R., Flanagan A.M. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. - DOI - PubMed
-
- Battaglia T.W., Mimpen I.L., Traets J.J.H., van Hoeck A., Zeverijn L.J., Geurts B.S., de Wit G.F., Noë M., Hofland I., Vos J.L., Cornelissen S., Alkemade M., Broeks A., Zuur C.L., Cuppen E., Wessels L., van de Haar J., Voest E. A pan-cancer analysis of the microbiome in metastatic cancer. Cell. 2024;187:2324–2335. doi: 10.1016/j.cell.2024.03.021. e19. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

