Quercetin activates autophagy in the distal ischemic area of random skin flaps through Beclin1 to enhance the adaptability to energy deficiency
- PMID: 39497976
- PMCID: PMC11533565
- DOI: 10.1016/j.heliyon.2024.e38181
Quercetin activates autophagy in the distal ischemic area of random skin flaps through Beclin1 to enhance the adaptability to energy deficiency
Abstract
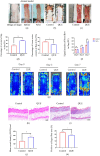
Random flaps are frequently employed in treating substantial skin abnormalities and in surgical tissue-rebuilding interventions. The random flap technique provides flaps of specific dimensions and contours to fit the surgical incision. However, blood supply deficiency and subsequent ischemia-reperfusion injury can cause severe oxidative stress and apoptosis, eventually leading to distal necrosis, which limits the clinical application of the flap. Quercetin (QUE) is primarily found in the glycoside form in many plant parts, such as stem bark, flowers, leaves, buds, seeds, and fruits. Cellular, animal, and clinical studies have demonstrated the antioxidant, anti-apoptosis, anti-inflammatory, and activation of autophagy properties of QUE. In previous studies, high doses of QUE effectively suppressed the survival of human umbilical vein endothelial cells (HUVECs) stimulated by hydrogen peroxide. However, different concentration gradients of QUE on HUVECs revealed a significant protective effect at a concentration of 10 mM. The protective impact of QUE on HUVECs was evaluated using scratch tests, CCK-8 assays, and EDU assays. Simultaneously, a mouse model of random skin flap was created, and the impact of QUE on skin flap survival was examined by intragastric injection. The QUE group showed a significantly larger survival area of the random flap and higher blood flow intensity compared to the control group. Furthermore, the beneficial effects of QUE were reversed by the autophagy inhibitor 3-MA. Therefore, autophagy plays a significant role in the therapeutic benefits of QUE on flap survival.
Keywords: Angiogenesis; Autophagy; ERK; Quercetin; Random flaps.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures







References
-
- Gaboriau H.P., Murakami C.S. Skin anatomy and flap physiology. Otolaryng Clin N Am. 2001;34(3):555. - PubMed
-
- Costeloe A., Newman J. Aesthetician role in facial plastic surgery and systemic therapy for healthy skin. Facial Plast Surg Cl. 2023;31(4):557–566. - PubMed
-
- Hyakusoku H., Gao J.H., Pennington D.G., Aoki R., Murakami M., Ogawa R. The microvascular augmented subdermal vascular network (ma-SVN) flap: its variations and recent development in using intercostal perforators. Brit J Plast Surg. 2002;55(5):402–411. - PubMed
-
- Park T.H. The utility of hyaluronidase for the free and pedicle flap salvage. J. Craniofac. Surg. 2023;34(3):1058–1060. - PubMed
-
- Wu L.J., Gao S.Y., Tian K., Zhao T.L., Li K. "Pingpong racket" flap model for evaluating flap survival. J Cosmet Dermatol-Us. 2021;20(8):2593–2597. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

