Synthetic bacterial consortia transplantation attenuates vaginal inflammation and modulates the immune response in a mouse model of Gardnerella vaginalis-induced bacterial vaginosis
- PMID: 39498013
- PMCID: PMC11533556
- DOI: 10.1016/j.heliyon.2024.e38218
Synthetic bacterial consortia transplantation attenuates vaginal inflammation and modulates the immune response in a mouse model of Gardnerella vaginalis-induced bacterial vaginosis
Abstract
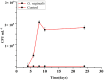
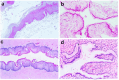
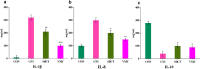
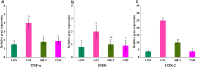
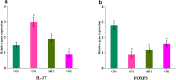
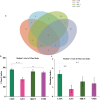
This study aimed to evaluate the efficacy of synthetic bacterial consortia transplantation (SBCT) and compare it with VMT (vaginal microbiota transplantation) in a mouse model of Gardnerella vaginalis-induced Bacterial vaginosis (BV). A murine model of G. vaginalis-induced BV was established, and mice were treated with SBCT, VMT, or saline. Histopathological changes, inflammatory cytokine levels, pro-inflammatory biomarker expression, helper T cell transcription factor expression, and vaginal microbiota composition were assessed. SBCT and VMT effectively suppressed G. vaginalis growth, reduced inflammation, and restored vaginal microbiota diversity. Both treatments attenuated epithelial damage, downregulated pro-inflammatory cytokines (IL-1β and IL-8), and upregulated the anti-inflammatory cytokine IL-10. SBCT and VMT also inhibited NF-κB activation, suppressed IL-17 expression, and enhanced Foxp3 expression in vaginal tissues. SBCT is a promising therapeutic approach for treating BV, as it effectively modulates the immune response and restores vaginal microbiota diversity in a mouse model of G. vaginalis-induced BV.
Keywords: Bacterial vaginosis; Gardnerella vaginalis; Immune response; Inflammation; Synthetic bacterial consortia transplantation; Vaginal microbiota transplantation.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Das S., Bhattacharjee M.J., Mukherjee A.K., Khan M.R. Recent advances in understanding of multifaceted changes in the vaginal microenvironment: implications in vaginal health and therapeutics. Crit. Rev. Microbiol. 2023;49:256–282. - PubMed
-
- Ahrodia T., Yodhaanjali J.R., Das B. Vaginal microbiome dysbiosis in preterm birth. Prog Mol Biol Transl Sci. 2022;192:309–329. - PubMed
LinkOut - more resources
Full Text Sources

