Targeted radionuclide therapy against GARP expressing T regulatory cells after tumour priming with external beam radiotherapy in a murine syngeneic model
- PMID: 39498075
- PMCID: PMC11533616
- DOI: 10.1016/j.heliyon.2024.e39543
Targeted radionuclide therapy against GARP expressing T regulatory cells after tumour priming with external beam radiotherapy in a murine syngeneic model
Abstract
Purpose: Radiation therapy (RT) exerts its anti-tumour efficacy by inducing direct damage to cancer cells but also through modification of the tumour microenvironment (TME) by inducing immunogenic antitumor response. Conversely, RT also promotes an immunosuppressive TME notably through the recruitment of regulatory T cells (Tregs). Glycoprotein A repetitions predominant (GARP), a transmembrane protein highly expressed by activated Tregs, plays a key role in the activation of TGF-β and thus promotes the immunosuppressive action of Tregs. The development of a theranostic approach targeting GARP combining imaging and targeted radionuclide therapy (TRT) was carried out.
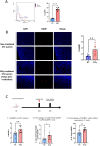
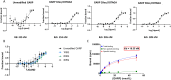
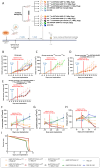
Methods: A preclinical model of 4T1 triple negative breast tumour-bearing BALB/c mice was used to show that GARP expression is increased after external beam radiation in the TME of our cancer model. We generated a theranostic probe through the bioconjugation of the chelating agent DOTAGA onto an anti-GARP monoclonal antibody. The bioconjugation with DOTAGA allows the radiolabelling of the DOTAGA-GARP conjugate with both Indium-111 for SPECT imaging and Lutetium-177 for TRT purposes.
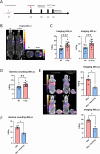
Results: We demonstrate that GARP expression is increased following RT in vivo and can be specifically detected and quantified using in vivo SPECT imaging with [111In]In-DOTAGA-GARP. In addition, 177Lu-DOTAGA-GARP limits tumour growth in our cancer model.
Conclusion: This theranostic strategy may allow for the personalization of cancer treatments by early detection of activated Tregs infiltration following RT and identification of patients likely to respond to Tregs-targeted therapy via TRT.
Keywords: External beam radiotherapy; GARP; SPECT imaging; T regulatory cells; Targeted radionuclide therapy.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. Ca - Cancer J. Clin. 2017;67:7–30. - PubMed
-
- Golden E.B., Apetoh L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 2015;25:11–17. - PubMed
-
- Shahabi V., Postow M.A., Tuck D., Wolchok J.D. Immune-priming of the tumor microenvironment by radiotherapy: rationale for combination with immunotherapy to improve anticancer efficacy. Am. J. Clin. Oncol. 2015;38:90–97. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

