Downregulation of aquaporins and PI3K/AKT and upregulation of PTEN expression induced by the flavone scutellarein in human colon cancer cell lines
- PMID: 39498081
- PMCID: PMC11532241
- DOI: 10.1016/j.heliyon.2024.e39402
Downregulation of aquaporins and PI3K/AKT and upregulation of PTEN expression induced by the flavone scutellarein in human colon cancer cell lines
Abstract
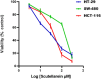
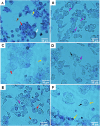
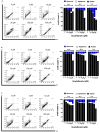
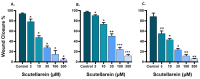
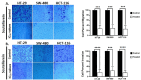
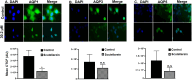
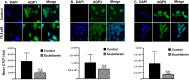
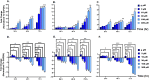
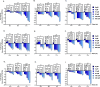
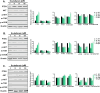
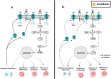
Scutellarein has an anticancer potential, but the pathway of its action has not been elucidated. This study investigated scutellarein efficacy against human colorectal cancer (CRC) and explored the possible pathway of its action. MTT assay was employed to detect scutellarein effect on HT-29, SW-480, and HCT116 cells viability. Scutellarein impact on programmed cell death was studied by Annexin V-FITC/PI and its role on migration and invasion was detected by wound healing and transwell chambers. Aquaporin (AQP) 1, 3, and 5 expression before and after scutellarein treatment was approached by quantitative polymerase chain reaction (RT‒qPCR) and immunostaining while Western blotting was used to explore scutellarein effect on PI3K/AKT pathway. Scutellarein induced apoptosis and necrosis in CRC cells, thus inhibiting proliferation, migration, and invasion. Colon cancer cells exhibited positive staining for AQP 1, 3, and 5 which was downregulated by scutellarein. PI3K/AKT pathway mediating cell proliferation and growth was also modulated by scutellarein; phosphatase and tensin (PTEN) was upregulated, whereas PI3K, AKT, and p-AKT expressions were downregulated, and the downstream mTOR and phosphorylated mTOR were also suppressed at the protein level. Data indicated that scutellarein suppressed growth, migration as well as invasion of these CRC cells by downregulating the expression of AQP 1, 3, and 5 and upregulating PTEN where the latter inhibited the genes and the proteins involved in PI3K/AKT pathway. The data indicate that scutellarein is a promising therapeutic agent that inhibits growth, migration, and invasion of CRC cells by down-regulating the expression of AQP 1, 3, and 5 and by PTEN up-regulation, thus inhibiting PI3K/AKT. These molecular alterations represent potential prognostic biomarkers for the metastasis of colon cancer, where the down-regulation of AQPs enhances patient survival.
Keywords: Apoptosis; Aquaporins; Colorectal cancer; Metastasis; PI3K/AKT signaling; PTEN; Scutellarein.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Berberine Inhibited Growth and Migration of Human Colon Cancer Cell Lines by Increasing Phosphatase and Tensin and Inhibiting Aquaporins 1, 3 and 5 Expressions.Molecules. 2023 Apr 29;28(9):3823. doi: 10.3390/molecules28093823. Molecules. 2023. PMID: 37175233 Free PMC article.
-
MicroRNA-126 Targeting PIK3R2 Inhibits NSCLC A549 Cell Proliferation, Migration, and Invasion by Regulation of PTEN/PI3K/AKT Pathway.Clin Lung Cancer. 2016 Sep;17(5):e65-e75. doi: 10.1016/j.cllc.2016.03.012. Epub 2016 Apr 6. Clin Lung Cancer. 2016. PMID: 27236384
-
Sinensetin flavone exhibits potent anticancer activity against drug-resistant human gallbladder adenocarcinoma cells by targeting PTEN/PI3K/AKT signalling pathway, induces cellular apoptosis and inhibits cell migration and invasion.J BUON. 2020 Mar-Apr;25(2):1251-1256. J BUON. 2020. PMID: 32521933
-
Hedyotis diffusa Willd inhibits proliferation and induces apoptosis of 5‑FU resistant colorectal cancer cells by regulating the PI3K/AKT signaling pathway.Mol Med Rep. 2018 Jan;17(1):358-365. doi: 10.3892/mmr.2017.7903. Epub 2017 Oct 26. Mol Med Rep. 2018. PMID: 29115462
-
miR-214 targets the PTEN-mediated PI3K/Akt signaling pathway and regulates cell proliferation and apoptosis in ovarian cancer.Oncol Lett. 2017 Nov;14(5):5711-5718. doi: 10.3892/ol.2017.6953. Epub 2017 Sep 15. Oncol Lett. 2017. PMID: 29113199 Free PMC article.
Cited by
-
Antitumor Effects of Quercetin and Luteolin in A375 Cutaneous Melanoma Cell Line Are Mediated by Upregulation of P-ERK, c-Myc, and the Upstream GPER.Life (Basel). 2025 Mar 7;15(3):417. doi: 10.3390/life15030417. Life (Basel). 2025. PMID: 40141761 Free PMC article.
-
Study of caspase-6 activity in aggressive HCT116 cells using methotrexate-encapsulated lactoferrin-conjugated solid lipid nanoparticles via in silico and in vitro approaches.Sci Rep. 2025 Jul 1;15(1):20775. doi: 10.1038/s41598-025-08089-w. Sci Rep. 2025. PMID: 40594879 Free PMC article.
-
Exploring Carboxamide Derivatives as Promising Anticancer Agents: Design, In Vitro Evaluation, and Mechanistic Insights.Int J Mol Sci. 2025 Jun 19;26(12):5903. doi: 10.3390/ijms26125903. Int J Mol Sci. 2025. PMID: 40565364 Free PMC article.
-
Repurposing Antiepileptic Drugs for Cancer: A Promising Therapeutic Strategy.J Clin Med. 2025 Apr 14;14(8):2673. doi: 10.3390/jcm14082673. J Clin Med. 2025. PMID: 40283503 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

