The considerations on selecting the appropriate decellularized ECM for specific regeneration demands
- PMID: 39498148
- PMCID: PMC11532911
- DOI: 10.1016/j.mtbio.2024.101301
The considerations on selecting the appropriate decellularized ECM for specific regeneration demands
Abstract
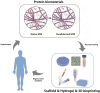
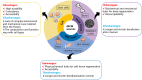
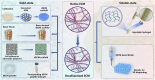
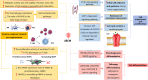
An ideal biomaterial should create a customized tissue-specific microenvironment that can facilitate and guide the tissue repair process. Due to its good biocompatibility and similar biochemical properties to native tissues, decellularized extracellular matrix (dECM) generally yields enhanced regenerative outcomes, with improved morphological and functional recovery. By utilizing various decellularization techniques and post-processing protocols, dECM can be flexibly prepared in different states from various sources, with specifically customized physicochemical properties for different tissues. To initiate a well-orchestrated tissue-regenerative response, dECM exerts multiple effects at the wound site by activating various overlapping signaling pathways to promote cell adhesion, proliferation, and differentiation, as well as suppressing inflammation via modulation of various immune cells, including macrophages, T cells, and mastocytes. Functional tissue repair is likely the main aim when employing the optimized dECM biomaterials. Here, we review the current applications of different kinds of dECMs in an attempt to improve the efficiency of tissue regeneration, highlighting key considerations on developing dECM for specific tissue engineering applications.
Keywords: Decellularized extracellular matrix biomaterials; Physicochemical properties; Tissue engineering; Underlying mechanisms on dECM-mediated regeneration process.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
We have nothing to declare.
Figures





References
-
- Griffin T.J., Cheung W.S., Zavras A.I., Damoulis P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006;77(12):2070–2079. - PubMed
-
- Brown M., Li J., Moraes C., Tabrizian M., Li-Jessen N.Y.K. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

